Using nanotechnology to give fuel cells more oomph
http://news.vanderbilt.edu/2016/08/using-nanotechnology-to-give-fuel-cells-more-oomph/[font face=Serif][font size=5]Using nanotechnology to give fuel cells more oomph[/font]
by David Salisbury | Aug. 8, 2016, 10:09 AM
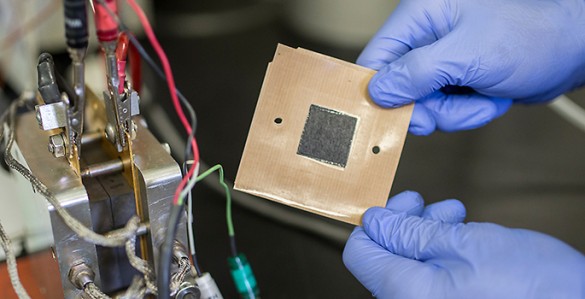
[font size=1]Nanofiber mat electrode (John Russell / Vanderbilt)[/font]
[font size=3]At the same time Honda and Toyota are introducing fuel cell cars to the U.S. market, a team of researchers from Vanderbilt University, Nissan North America and Georgia Institute of Technology have teamed up to create a new technology designed to give fuel cells more oomph.
The project is part of a
$13 million Department of Energy program to advance fuel cell performance and durability and hydrogen storage technologies announced last month.
The $4.5 million collaboration is based on a new nanofiber mat technology developed by Peter Pintauro, the H. Eugene McBrayer Professor of Chemical Engineering at Vanderbilt, that replaces the conventional electrodes used in fuel cells. The nanofiber electrodes boost the power output of fuel cells by 30 percent while being less expensive and more durable than conventional catalyst layers. The technology has been patented by Vanderbilt and licensed to Merck KGaA in Germany, which is working with major auto manufacturers in applying it to the next generation of automotive fuel cells.
…
Conventional fuel cells use thin sheets of catalyst particles mixed with a polymer binder for the electrodes. The catalyst is typically platinum on carbon powder. The Vanderbilt approach replaces these solid sheets with mats made from a tangle of polymer fibers that are each a fraction of the thickness of a human hair made by a process called electrospinning. Particles of catalyst are bonded to the fibers. The very small diameter of the fibers means that there is a larger surface area of catalyst available for hydrogen and oxygen gas reactions during fuel cell operation. The pores between fibers in the mat electrode also facilitate the removal of the waste water. The unique fiber electrode structure results in higher fuel cell power, with less expensive platinum.
…[/font][/font]
