Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Environment & Energy
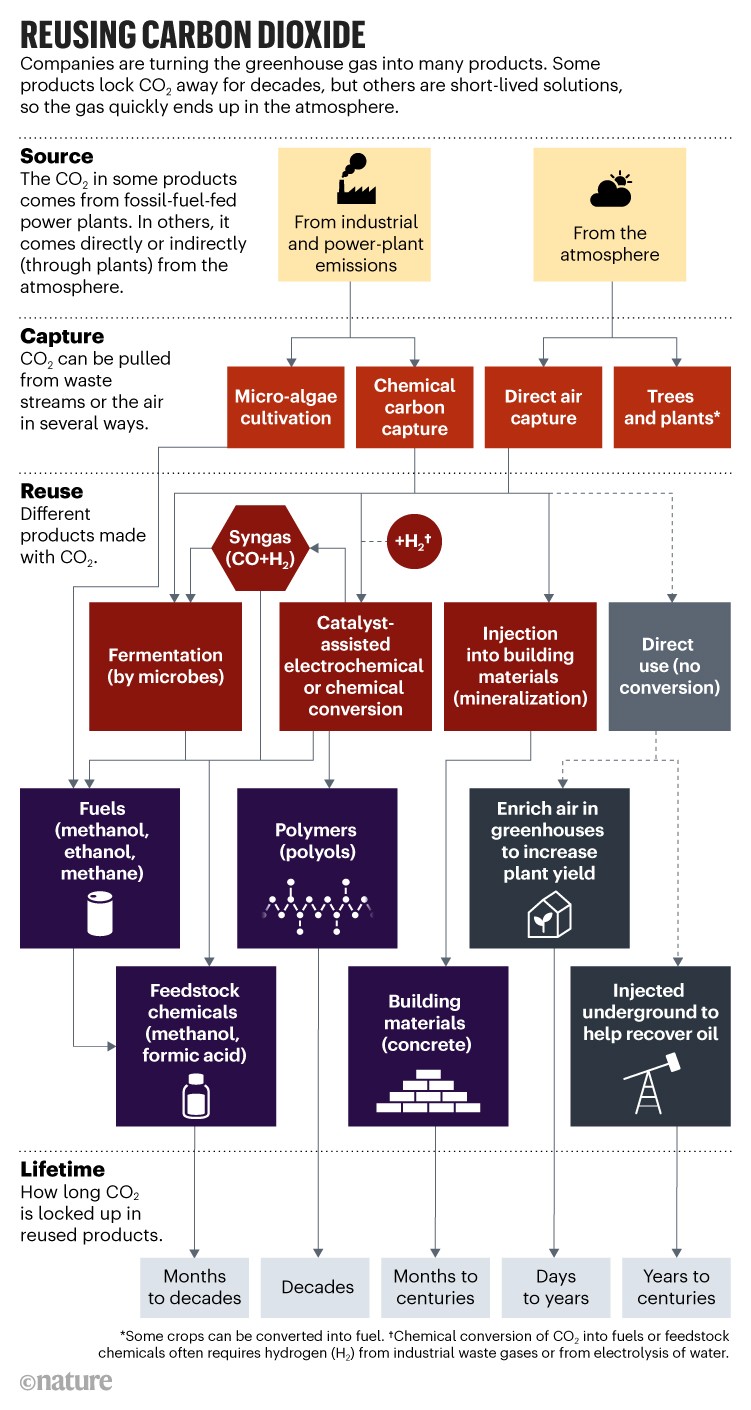
Related: About this forumA graphic on "making stuff out of CO2" and the time scale of sequestration by "stuff."
This came into my Nature Briefing email some weeks back and I never got around to posting it. The general idea is one of which I'm quite fond, but the graphic calls my enthusiasm in question, at least to some extent.
It's from here: The race to upcycle CO2 into fuels, concrete and more
Subtitle:
Companies are scrambling to turn the greenhouse gas into useful products — but will that slow climate change?
A graphic from it.
Some excerpts:
Tongyezhen is a town with coal in its bones. In this part of China’s Henan province, people have been mining coal and smelting metals for millennia. Today, Tongyezhen hosts a sprawling industrial park where huge ovens bake coal and limestone into coke and lime, both key ingredients for producing steel. Unsurprisingly, it is one of the smoggiest places in China.
It might seem an unlikely venue for a clean-technology milestone. But later this year, a chemical plant here is set to become the world’s largest facility for recycling carbon dioxide into fuel. It will combine CO2 from a lime kiln with excess hydrogen and CO2 from a coking furnace to produce methanol, an industrial chemical used for fuel and to make plastics. Carbon Recycling International (CRI), the Reykjavik-based firm behind the operation, says that the Tongyezhen plant will recycle about 160,000 tonnes of CO2 per year — equivalent to the emissions from tens of thousands of cars — that would otherwise go into the atmosphere.
It’s an alluring idea: industrial CO2 emissions are warming the climate, and many countries are working on capturing the gas and storing it underground. But why not recycle it into products that are both virtuous and profitable? As long as the recycling process avoids creating more carbon emissions — by using renewable energy, or excess resources that would otherwise be wasted — it can reduce the CO2 that industry pumps into the atmosphere and lower the demand for fossil fuels used in manufacturing. That’s a double climate win, proponents say.
This kind of recycling (sometimes called upcycling) is an increasingly crowded field, as companies big and small race to market a bewildering array of products made from CO2. Some are boutique items for the climate-conscious shopper — vodka or diamonds, for example — but most are staples of the global economy: fuels, polymers, other chemicals and building materials. More than 80 firms are working on new approaches to using CO2, noted a 2021 report by Lux Research, a market-research company in Boston, Massachusetts. The market for these products is tiny today, amounting to less than US$1 billion — but Lux predicts that it will grow to $70 billion by 2030, and could reach $550 billion by 2040...
...But there are tough questions about whether CO2 recycling genuinely benefits the climate. Many of the products made this way only briefly delay carbon’s journey into the atmosphere — fuels are burnt, products made from chemicals degrade and the CO2 consumed during their creation is released again. That will happen at Tongyezhen: much of the methanol produced is destined to be burnt as fuel in China’s growing fleet of methanol-powered vehicles.
Meanwhile, some estimates suggest that the global market for recycled CO2 products is unlikely to lock up more than a few per cent of the CO2 that humans release into the atmosphere by burning fossil fuels, which totalled 36 billion tonnes last year. CRI’s plant, for one, will convert the equivalent of a little over 2 minutes’ worth of annual global CO2 emissions. “We can avoid a lot of that, for a lot less money, than we can by turning CO2 into stuff,” says Niall Mac Dowell, an energy-systems engineer at Imperial College London.
“The assumption that we can fix this climate-change problem in an economically attractive and easy way — at best it’s naive, and at worst it’s actively disingenuous,” he says. It’s an argument that’s heating up as CO2 recycling goes mainstream....
It might seem an unlikely venue for a clean-technology milestone. But later this year, a chemical plant here is set to become the world’s largest facility for recycling carbon dioxide into fuel. It will combine CO2 from a lime kiln with excess hydrogen and CO2 from a coking furnace to produce methanol, an industrial chemical used for fuel and to make plastics. Carbon Recycling International (CRI), the Reykjavik-based firm behind the operation, says that the Tongyezhen plant will recycle about 160,000 tonnes of CO2 per year — equivalent to the emissions from tens of thousands of cars — that would otherwise go into the atmosphere.
It’s an alluring idea: industrial CO2 emissions are warming the climate, and many countries are working on capturing the gas and storing it underground. But why not recycle it into products that are both virtuous and profitable? As long as the recycling process avoids creating more carbon emissions — by using renewable energy, or excess resources that would otherwise be wasted — it can reduce the CO2 that industry pumps into the atmosphere and lower the demand for fossil fuels used in manufacturing. That’s a double climate win, proponents say.
This kind of recycling (sometimes called upcycling) is an increasingly crowded field, as companies big and small race to market a bewildering array of products made from CO2. Some are boutique items for the climate-conscious shopper — vodka or diamonds, for example — but most are staples of the global economy: fuels, polymers, other chemicals and building materials. More than 80 firms are working on new approaches to using CO2, noted a 2021 report by Lux Research, a market-research company in Boston, Massachusetts. The market for these products is tiny today, amounting to less than US$1 billion — but Lux predicts that it will grow to $70 billion by 2030, and could reach $550 billion by 2040...
...But there are tough questions about whether CO2 recycling genuinely benefits the climate. Many of the products made this way only briefly delay carbon’s journey into the atmosphere — fuels are burnt, products made from chemicals degrade and the CO2 consumed during their creation is released again. That will happen at Tongyezhen: much of the methanol produced is destined to be burnt as fuel in China’s growing fleet of methanol-powered vehicles.
Meanwhile, some estimates suggest that the global market for recycled CO2 products is unlikely to lock up more than a few per cent of the CO2 that humans release into the atmosphere by burning fossil fuels, which totalled 36 billion tonnes last year. CRI’s plant, for one, will convert the equivalent of a little over 2 minutes’ worth of annual global CO2 emissions. “We can avoid a lot of that, for a lot less money, than we can by turning CO2 into stuff,” says Niall Mac Dowell, an energy-systems engineer at Imperial College London.
“The assumption that we can fix this climate-change problem in an economically attractive and easy way — at best it’s naive, and at worst it’s actively disingenuous,” he says. It’s an argument that’s heating up as CO2 recycling goes mainstream....
With due respect to Dr. Mac Dowell, it's quite possible that he may be missing some things, as is the chart above, since both rely on some assumptions about energy as well as carbon sources.
If the carbon is captured from the environment - direct air capture while possibly viable seems from my perspective to be the least likely option, but I hold some respect to dry reforming of some biomass, as well as seawater capture (about which I am currently writing) the carbon cycle is closed and there is no need to dump 36 billion tons of carbon dioxide directly into the atmosphere.
Secondly, there is no mention of metal carbides, nor of carbon fibers, nor any of the highly useful allotropes of carbon, nanotubes and teh like. (I expect metal carbides - steel is, in a sense, an example, although the carbon source is currently coal - to play a big role in any sustainable economy which may exist.
On the first point the article continues a little further on:
Life-cycle arguments
Whether products recycled from industrial CO2 emissions actually protect the climate is unclear — because the CO2 they capture will still be released into the atmosphere if the molecules are burnt or broken down. Drawing CO2 directly from the atmosphere could have clearer climate benefits, but capturing the gas from air is extremely expensive, as are products made that way.
Proponents argue that recycling industrial CO2 into chemicals can reduce emissions in another way — by avoiding some fossil-fuel-based production. “Our process helps keep fossil fuels in the ground by tapping into existing streams of CO2,” a spokesperson for Twelve told Nature.
The stringent way to examine this is through a life-cycle analysis (LCA) — a detailed accounting of the carbon involved in making and using a product, from the origins of its CO2 to its final fate. Many CO2-recycling firms say they have done these audits, but don’t publish them because they contain proprietary information...
Whether products recycled from industrial CO2 emissions actually protect the climate is unclear — because the CO2 they capture will still be released into the atmosphere if the molecules are burnt or broken down. Drawing CO2 directly from the atmosphere could have clearer climate benefits, but capturing the gas from air is extremely expensive, as are products made that way.
Proponents argue that recycling industrial CO2 into chemicals can reduce emissions in another way — by avoiding some fossil-fuel-based production. “Our process helps keep fossil fuels in the ground by tapping into existing streams of CO2,” a spokesperson for Twelve told Nature.
The stringent way to examine this is through a life-cycle analysis (LCA) — a detailed accounting of the carbon involved in making and using a product, from the origins of its CO2 to its final fate. Many CO2-recycling firms say they have done these audits, but don’t publish them because they contain proprietary information...
This of course, represents energy storage, which is always conducted at a thermodynamic loss. This said, if the storage results from the storage of energy that would otherwise be rejected to the atmosphere - this requires high temperatures and modern refractory materials - it may make sense.
Intriguing is this open sourced paper from open sourced Science Advances: Asmita Jana and Taishan Zhu and Yanming Wang and Jeramie J. Adams and Logan T. Kearney and Amit K. Naskar and Jeffrey C. Grossman and Nicola Ferralis, Atoms to fibers: Identifying novel processing methods in the synthesis of pitch-based carbon fibers. Science Advances , 8, 11,eabn1905, 2022.
This paper discusses the fact that carbon fibers, while extremely useful, currently are 4 times as expensive as aluminum, and details some process details that make it so. The current process begins with the polymerization of acrylonitrile, itself obtained from dangerous fossil fuels in the form of propene and ammonia which is made from hydrogen and nitrogen gas, the bulk of the world's hydrogen in turn also being produced from dangerous fossil fuels.
However a process is known to produce propene from methanol is well known: Yarulina, I., De Wispelaere, K., Bailleul, S. et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nature Chem 10, 804–812 (2018). It is well known that methanol can be synthesized from the hydrogenation of, um, carbon dioxide. (In fact, about 10% of the world's hydrogen, currently is devoted to methanol synthesis, as I noted in another thread: The current sources and uses of hydrogen.) Again, most of the world's hydrogen is currently produced from dangerous fossil fuels, but it is possible to engage in clean hydrogen production utilizing thermochemical water splitting, with a clean primary energy source, of which there is only one, nuclear energy.
From the Science Advances paper, it is clear that another driver of the cost of carbon fibers is heat, and thus high temperature exchange networks theoretically, and likely practically, can be used to reduce this heat cost via high thermodynamic efficiency.
It thus seems conceivable that the cost of carbon fibers may be well significantly released. Nor is the point of the Science Advances paper unworthy of consideration, the management of pitch. Pitch is generally a dangerous fossil fuel product consisting of a highly heterogenous and structurally complex set of molecules falling into a class called "asphaltenes." Much recent work utilizing very high resolution mass spectrometry - that of FT-ICR-MS - has been devoted to understanding the structure of asphaltenes. It is also true that asphaltenes are often a side product of the pyrolysis of biomass, and/or plastic wastes. This suggests yet another route to carbon fibers that depends on carbon dioxide effectively removed from the air. Structural elucidation is the first step on the way to specifications, and specifications define the goals of process chemistry and allow for its development.
I therefore suspect that perhaps, again, it is Dr. Mac Dowell who may be guilty of being disingenuous. With clean, sustainable primary energy - again there is one form and only one form, nuclear energy - carbon utilization in economically viable ways certainly seems promising.
We shall see.
From my perspective carbon dioxide utilization is far superior to carbon dumps. All of our pursuits of addressing climate change have failed, but some are worthy of further evaluation and research and some are not. The article and the chart above have not left me without a sense of some hard won optimism.
The key to this problem is, in my view, materials science, a subject in which a golden age may well emerge. We should hope so.
I trust you will have a nice evening.
InfoView thread info, including edit history
TrashPut this thread in your Trash Can (My DU » Trash Can)
BookmarkAdd this thread to your Bookmarks (My DU » Bookmarks)
0 replies, 707 views
ShareGet links to this post and/or share on social media
AlertAlert this post for a rule violation
PowersThere are no powers you can use on this post
EditCannot edit other people's posts
ReplyReply to this post
EditCannot edit other people's posts
Rec (3)
ReplyReply to this post