Environment & Energy
Related: About this forumThe Energy Required to Supply California's Water with Zero Discharge Supercritical Desalination.
Last edited Sun Jun 5, 2022, 05:50 PM - Edit history (2)
The paper I'll discuss in this post is this one: Surika van Wyk, Aloijsius G.J. van der Ham, Sascha R.A. Kersten, Analysis of the energy consumption of supercritical water desalination (SCWD), Desalination, Volume 474, 2020, 114189.
The paper seems to be open sourced; anyone can read it. Therefore I will not excerpt it much except to refer to the general conception.
The basis of this technology is that beyond the critical point of water, which is at temperatures at or above 374°C and pressures at or above 22.1 MPa, salts are not soluble in water, and in the case of seawater, it separates into two phases, one consisting essentially of pure water, and another that is basically salts. Separation of these phases by physical means provides fresh water.
I discussed some issues with supercritical water in a relatively recent previous post in this space: Experimental Supercritical (Sea)Water Oxidation of Microplastics To Yield Hydrogen and Other Gases.
At that time, it slipped my mind that I had in my files a paper that had a very nice representation of the three dimensional phase diagram, not of supercritical seawater, but the closely related sodium chloride water system. Here it is:
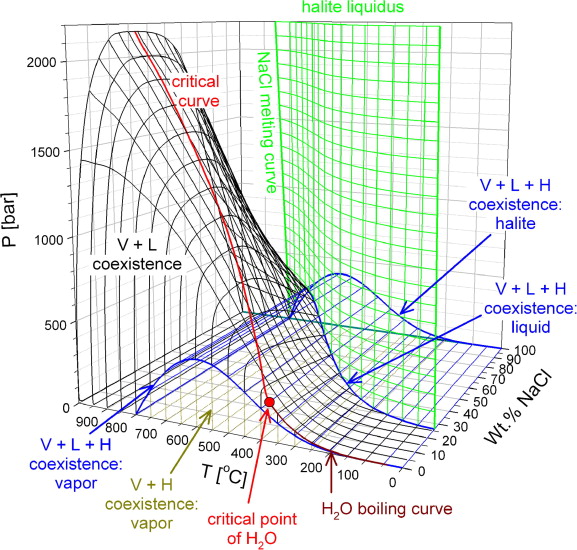
The caption:
The source: Thomas Driesner, Christoph A. Heinrich, The system H2O–NaCl. Part I: Correlation formulae for phase relations in temperature–pressure–composition space from 0 to 1000°C, 0 to 5000bar, and 0 to 1 XNaCl, Geochimica et Cosmochimica Acta, Volume 71, Issue 20, 2007, Pages 4880-4901.
Heating seawater to these temperatures under this much pressure obviously requires energy.
As most of us know, the State of California is experiencing severe drought - it is a widely held suspicion that climate change will make it more or less permanent - but it is a coastal State and in theory it can (and in places does) desalinate seawater. All desalination requires the expenditure of energy, but most of them are not zero discharge. The release concentrated brines back into the ocean, leading to highly saline zones which, if near the intakes, raise the energy cost of desalination and in all cases of brine dumping, impact local ecosystems.
Supercritical desalination by contrast, is "zero discharge." The salts are either molten or solid; depending on temperature they may also have a significant vapor pressure. The salts can then recovered, depending on conditions in a relatively purified form, and then be either sold for use elsewhere or utilized at the site at which they are obtained for a number of processes.
A third phase, not shown in the phase diagram above, is a gas/supercritical phase. As mentioned in my earlier thread, this phase will largely consist of carbon dioxide, hydrogen, and a few other gases.
Seawater contains considerable carbon, both represented by living plankton and regrettably in modern times bulk, micro, and nano plastics, and in an inorganic form, dissolved CO2 which is then hydrated into the weak acid H2CO3, which at the pH of seawater, currently around 8.1, is deprotonated to give the bicarbonate ion HCO3-, and carbonate CO3=. The ocean is, as most people know, the major sink for the dangerous carbon dioxide waste we dump each year while we all wait, increasingly breathlessly for the grand so called "renewable energy" nirvana that hasn't come and isn't here, and, in my opinion, so far as the belief that it will come, is rather like waiting for Jesus to come back to judge the living and the dead.
The concentration of carbon in seawater is so much higher than that of air that I personally belief that any successful effort by future generations to clean up the carbon mess my generation, which has been entirely contemptuous of the planet and all future generations to live on it, has made.
History will not forgive us; nor should it.
An excellent open sourced reference on inorganic carbon is available on line from a scientist, Andrew G. Dickenson, at the famous Scripps Institute of Oceanography in San Diego, just down the road from where I lived many, many, many years ago: INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER I am going to use the figures for the concentration of the three CO2 species found on the relevant page of the presentation which reads "ACID-BASE SPECIES COMPRISE A SUBSET OF THE “MINOR CONSTITUENTS” PRESENT IN SEAWATER." I am going to use the concentrations found on that page for μmoles kg^(-1) seawater, respectively 1900 μmoles kg^(-1) HCO- seawater, 158 μmoles kg^(-1) HCO- and 15.5 μmoles kg^(-1) solvated CO2. This totals to about 2.1 mmol of carbonate species. Thus, carrying the calculation through for the molecular weight of each species, and the percentage of elemental carbon in each of the inorganic carbonate species, a kg of seawater contains about 25.3 mg of inorganic carbon for each kg of seawater. That doesn't sound like much until one considers the bulk weight of the ocean, and for that matter, the water demand of the drying State of California.
(I once lived in close proximity to the Scripps Institute of Oceanography, my relationship with that institution consists largely of having driven past it in a car spewing the dangerous fossil fuel waste carbon dioxide out of its tailpipe.)
By the way, the parent website for this Dickenson presentation linked above if that of the International Atomic Energy Agency. I have no idea why this is so, but in my view, this agency is the only agency seriously working to save the world from itself. I have never deviated from my position that nuclear energy represents the only option to address climate change, to save what can still yet be saved, and restore what might be restored.
Here is a graphic from the Dickenson presentation which nicely shows the fractional constituents of seawater:

Unsurprisingly it's mostly water. The carbonate, and for that matter the uranium, copper, zinc, and other elements, most notably phosphorous, an essential element for life and one that is rapidly being exhausted by mining, are all contained in the "minor constituents" box, 240 mg/kg combined.
What may be of particular interest in a discussion of zero discharge supercritical water desalination will be magnesium, but we should also consider the fate of sodium chloride. It may be that this material will be essential for some level of geoengineering if, as seems likely, climate change shuts down the Gulf Stream, greatly destabilizing (further) the weather of Europe as well as parts of the United States.
So how much water does California use?
Here, from an internet web page from the "non-partisan" Public Policy Institute of California is a nice graphic breakdown of the water demand in California in wet and in dry years, simple enough, I hope, for even an anti-nuke (i.e. few brains and not much in the way of education or ethics) to understand:

For calculations here, I am going to use the 104 million acre-feet figure in this graphic since the point is to address unrestricted urban and agricultural water in the State, while freeing up all the water to maintain or restore that which has been threatened or destroyed by water diversion in the State. What is not listed here, in terms of the categories listed is fracking water, which is very popular with at least one anti-nuke here but which I oppose categorically. (Arguably supercritical water might be used to restore ground water destroyed by fracking while we all waited for the grand "renewable energy" nirvana that didn't come, isn't here, and won't come, but I'll ignore that for the time being.)
First let's get rid of the pixilated unit "acre-feet" by converting it to the SI unit m^3. The conversion factor is 1233.4 m^3/acre-foot. Thus a demand of 104 "acre-feet" is 128 billion m^3. Of this, urban demand is 10.3 billion m^3; agricultural demand is 37.2 billion M^3, for a grand total of 47.5 billion m^3.
Although the paper is open sourced, for convenience, let me excerpt the paper cited at the outset:
...Already threatening ecosystems...
Recently we had here at DU lots of delusional cheering for 20 minutes of nearly "100% renewable energy" in the State of California on a Sunday afternoon at a time low demand, the sun shining and the wind blowing.
California 100 percent powered by renewables for first time
It is useful to look at the CAISO power demand and supply curves on May 1, 2022, the day before these minutes (literally) of "100% Renewable" California power took place.
Demand:

Supply:

The "100% renewable" figure, according to the newspaper article cited in the "California 100 percent powered by renewables for first time" DU post that generated so many cheers was at 2:45 in the afternoon. Note that the "100% renewable energy" claim is somewhat disingenuous; at no point did California stop producing electricity by burning dangerous natural gas and dumping the waste directly into the planetary atmosphere, although it was exporting electricity to other states. By 7 pm (19:00), the largest source of electricity to the grid in the State was provided by burning dangerous natural gas and dumping the waste directly into the planetary atmosphere.
It now behooves me to compare May, 1, 2022 with June 18, 2021, when California was experiencing extreme temperatures.
Indio is in the Imperial Valley, a major agriculture area, the "salad bowl" of America; the agriculture is supported by irrigation using the rapidly dying Colorado River where all time lows (since the 1930's when it was built) in the level of "Lake" Mead behind the Hoover Dam are being recorded, not by "...by 'such and such year,'" but now. This is not going to be a good year for cheap salads.
Bakersfield's high was 109°F, (43°C) at 17:54 PTD (5:54 pm). This is the same temperature used by the researchers testing human heat endurance in the reference and text I will repeat below from the earlier post. Temperatures in that city were at or above 100 °F, (37°C) from 11:00 PDT to to 18:00 PDT (6:00 PM).
Lancaster's high on 06/18/21 was 109°F, (43°C), recorded at 15:40 PDT (3:40 PM). Temperatures in that city were at or above 100 °F, (37°C) from 9:45 PDT to to 17:56 PDT (5:56 PM).
CA Extreme Temperatures, Electricity Demand Peaks, Timing of So Called "Renewable Energy" peaks.
I have redone the graphics found in that post using the CAISO site for cleanliness.
Demand:

Supply:

From the "CA Extreme Temperatures" called "renewable energy" on that day:

For a period of 12 hours on that day, where California was experiencing temperatures in various parts of the State that could kill a person without access to air conditioning - and probably did kill people albeit not so reported - the wind industry in the entire State, spread over more than 1500 square miles was producing less electricity than the Diablo Canyon Nuclear Plant was producing in two buildings on less than 15 acres of land. The highest demand on 6/18/21 was more than double the low demand seen on a Sunday afternoon when for a period of about half an hour, it is alleged that all of California's electricity was provided by so called "renewable energy," resulting in delusional cheering.
I take this diversion this because water & energy are intimately connected. The Diablo Canyon nuclear plant depends on access to water; it is a functional desalination plant, albeit not a desalination plant of the type I will discuss: It discharges brine to the ocean. Climate change, driven by decisions like those made in California to burn dangerous natural gas and dump the waste directly into the planetary atmosphere, also effects obviously access to water. We are seeing terrifying things in the American West with respect to water, not in some imagined future where climate becomes a problem, but now. This long anticipated future climate disaster has arrived. Again, and again, and again: NOW!.
Supercritical water is a very high energy substance, and the point of this post is to consider it. An important point is that one cannot produce supercritical water in spurts and stops. It's generation needs to be continuous. The best environmentally and economically sustainable systems are all continuous.
The purpose of the paper was to evaluate a very particular approach to desalination, which is to exploit the lower heat capacity of salt when compared to seawater, that is to use feed water that is saltier than seawater. (Seawater has a salinity of about 35 g salt/kg.)
Here, for convenience, are tables 1 and 2 from the paper, giving the energy demand for the utilization of the high salinity scheme described in the paper (and concentrations modeling seawater), Table 1, as well as some comparators from the literature, Table 2.


This covers some possible states. For the calculations I will apply to California, with the understanding that these are not set in stone, I choose to use 450 MJ/kg that is described in this paper, as the Dutch authors have actually built a small pilot plant and operated it:
Surika van Wyk, Samuel O. Odu, Aloijsius G.J. van der Ham, Sascha R.A. Kersten, Design and results of a first generation pilot plant for supercritical water desalination (SCWD), Desalination, Volume 439, 2018, Pages 80-92.
The schematic of the design is shown:

One reason for choosing this particular iteration is that the temperature is higher than those in the other paper: The unit has a maximum temperature of 500 °C; experimentally it operated at 430 °C.
Personally I would rather see temperatures closer to the melting point of sodium chloride, which is 800 °C; rather than a cyclone, a device for separating solids from fluids, I would prefer a kind of knock out drum for fluid phase separation. Fluid phases allow for continuous processing. The desalination system in my vision would be the third component in a heat exchanger network, the source heat of which would be set to be around 1400 °C, the temperature at which Ce(IV)oxide decomposes to give Ce(III) oxide and oxygen: 2CeO2 ↔ Ce2O3 + O2.
The systems in these papers use the heat of the supercritical fresh water to preheat the brines (or seawater), whereas I wouldn't do that, I'd utilize gaseous heat flows from the previous two components of the heat network. In my vision, one would recover the energy in the supercritical water for the purpose of pumping: The coast of California is separated from it's largest agricultural valleys, the Imperial and the San Joaquin by the coastal mountain ranges. Indeed, such a system might be designed as a long range heat pump, allowing for the temporary storage of thermal energy as opposed to less efficient (but more popular in the public mind) energy storage systems such as hydrogen and/or, even worse, batteries.
As this stuff is visionary, it's easy to say: It's quite another thing to design and build such a system, but as my son is an engineer, maybe I can leave him with these aspirational ideas.
So let's cut to the chase, assuming the equivalent of a wet year. I've done the calculation off line in a spreadsheet:
At 450 MJ/m^3, meeting the urban demand of around 10 billion cubic meters of water would amount to about 4.6 Exajoules of energy.
For the 37 billion cubic meters of agricultural water utilized in a wet year, around 16.7 Exajoules of energy would be required.
Thus we are talking about 20 to 21 Exajoules.
For comparison purposes, the entire United States is currently running at about 95-100 Exajoules per year.
It goes without saying that the heat networks involved would produce lots of energy in other forms.
"Green" California, which advertises itself as running on so called "renewable energy" - destroyed ecosystems be damned (or dammed) - consumed, in 2019, according to the EIA 2218.7 Trillion BTU of energy provided by dangerous natural gas. In civilized units this works out to 2.341 Exajoules.

(The graphic is interactive at the website.)
According to the Engineering Toolbox, a kg of dangerous natural gas (mostly methane) contains about 52MJ/kg. This translates to 45 million tons of gas. Assuming that natural gas is mostly methane, with a molecular weight of roughly 16 grams/mol, and that carbon dioxide is roughly 44 grams/per mole, this suggests that the amount of the dangerous fossil fuel waste carbon dioxide dumped more or less irretrievably by California's dangerous natural gas consumption was about 124 million tons in 2019.
Above, we saw that a liter of seawater contains about 2.1 mmol/liter of inorganic carbon (in the form of carbonates). A cubic meter contains 1000 liters, suggesting 2.1 moles of carbon per cubic meter.
CO2 is not particularly soluble in supercritical water and thus there will be a third phase in the system, a gas (actually supercritical) CO2 phase which, from the supercritical water oxidation of biomass (plankton) and nano, micro, and macro plastics in the water, some hydrogen as well. The inorganic carbon content of 47.5 billion cubic meters of seawater is roughly 100 million moles of carbon, which translates, as CO2, to around 4.4 million tons of carbon, not all the impressive.
There is, however, more carbon available in sea water as "particulates" The amount varies widely from region to region, it is probably the case that the organic carbon, both DOM (dissolved organic matter) and plankton suggest that the carbon dioxide recovered would, in fact, be significant. Organic carbon content of seawater can be, and is, monitored by satellite. Here, for example, is a paper describing calibration of the colorimetric procedure: Le, C., Zhou, X., Hu, C., Lee, Z., Li, L., & Stramski, D. (2018). A color-index-based empirical algorithm for determining particulate organic carbon concentration in the ocean from satellite observations. Journal of Geophysical Research: Oceans, 123, 7407–7419. Conveniently, the calibrations were conducted off the coast of Southern California and Northwest Mexico and are shown graphically in figure 7 of the open sourced paper:

The caption:
We note that the coast regions of the ocean, particularly around the cities - the area between the Channel Islands and Los Angeles in particular, but everywhere along the coast - are deep red in color, corresponding to 200 mg of organic carbon particulates per cubic meter. Surprisingly - at least surprising to me - is that particulates would not add all that much to the recovery of carbon even at 200 mg/m^3, would only recover a few tens of thousand metric tons of carbon, if that, perhaps a little more if seaweeds were harvested. A great deal has been written about the use of algae to concentrate carbon from the air; the major problem being the energy required to remove water. It should be obvious that high temperature supercritical water with a suitable heat transfer barrier would be a useful tool for drying, with the caveat that algae requires both phosphorous (albeit available from waste water) fixed nitrogen and most importantly water, the topic at hand.
An important point, nonetheless is that supercritical water is an excellent tool for reforming and producing syngas from carbon containing materials. Under these conditions all of the microplastics, now found everywhere in the ocean and indeed in almost ever seaborne organism, would be oxidized to carbon dioxide and hydrogen, syngas. In addition, phosphorous, which probably accounts to the high particulate carbon readings near the coast owing to sewage outfall pipes and agricultural and landscaping run off would be recovered.
As of 2019, California "disposed" of 48.6 million tons of municipal trash, while it "diverted" a further 28.6 million tons were said to be recycled, of which 14.4 million tons were "recycled" using "seaborne transport." (I would argue that no one actually knows the fate of that 14.4 million tons; call me a cynic if you will.) It reportedly works out to about 6.7 kg of waste per person per day.
An interesting paper by South African Scientists with a Canadian co-author, An overview of factors affecting the rate of generation and Physical Composition of Municipal Solid Waste O.A. Adeleke et al 2021 IOP Conf. Ser.: Mater. Sci. Eng. 1107 012096 argues that the volume and composition of solid waste is a function of wealth.
California is, of course, a relatively wealthy state, and may remain so with access to water. It maintains a web page devoted to describing the composition of its waste stream: Waste Characterization, Residential Stream. Plugging in Los Angeles Country as a sample, one can see that about 75% of the municipal waste there consists of carbon compounds, including paper and paper related materials like cardboard, roughly 20%, plastics, another 10%, food waste, yard waste, textiles and other sundry organic waste, 45%. (This is not dramatically different than the waste profile of Johannesburg described by Adeleke as cited above.) Rather than be involved in the largely failed practice, with it's subtle but real energy requirements and inefficiencies, of household waste "recycling," a single, "one size fits all" waste recycling program of recycling the carbon of the waste stream via wet (supercritical water) or dry (supercritical carbon dioxide, a "reverse Allam cycle" ) reforming seems worthy of recommendation. This reforming produces the mixture referenced above, syngas, a mixture of carbon oxides and hydrogen.
California is currently laced with pipelines for dangerous natural gas, which it largely burns, again, dumping the waste directly into the planetary atmosphere, most recently recorded, as stated above, at a rate of around 124 million metric tons/year. The heating value of dangerous natural gas is said to be between 42 and 55 MJ/kg, that of the wonder fuel dimethyl ether, DME, is 29 MJ/kg.
The wonder fuel DME is prepared by hydrogenation of carbon dioxide.
This reaction is exothermic, it releases energy. Industrially, DME is not generally manufactured in the single stage reaction described above, since the heat generated tends to reduce the lifetime of the catalysts required, generally copper based, but rather in a two stage reactions:

Source: Unravelling Proximity-Driven Synergetic Effect within CIZO–SAPO Bifunctional Catalyst for CO2 Hydrogenation to DME Libo Yao, Xiaochen Shen, Yanbo Pan, and Zhenmeng Peng, Energy & Fuels 2020 34 (7), 8635-8643. (This paper proposes a catalyst for a single stage reaction; similar catalysts for single step CO2 to DME conversions are also under consideration for the preparation of this valuable fuel.)
If free of the problematic hydrogen from which it is synthesized, the most important feature of DME is that it can be more or less dropped directly into existing dangerous natural gas infrastructure, and in fact, all LPG infrastructure. (Over the decades, one might have seen millions of wishful thinking commentaries on a putative, "hydrogen economy." In the real world this hyped nonsense should have been dismissed years ago; regrettably one can still see it, here and elsewhere. The planet cannot afford the replacement of all of the industrial infrastructure to satisfy ever more absurd fantasies.)
The advantages of DME over dangerous natural gas, and in fact, LPG, gasoline, diesel fuel and in fact, many refrigerants, are enormous, the most important being it's extremely low climate forcing potential in comparison to the chief component of dangerous natural gas, methane. The atmospheric half-life of DME is about 5 days, compared to decades for methane, and centuries for refrigerants.
If the heat released in the synthesis of DME is recovered by preheating seawater on the way to the supercritical state, after the newly synthesized gas has expanded against a turbine to generate electricity and/or to drive compressors for the simple liquefaction of DME - it's critical temperature is well above the boiling point of water - the regasification will absorb heat, that is provide cooling. The implications should be obvious, which is to give California the capability of shipping for export, for internal use at a distance, and/or thermodynamically superior storage, heat, albeit probably not prodigious amounts.
The side product of this scheme, on which I have focused little, is electricity. While noting the amount of energy for supercritical water desalination for all of the urban and agricultural demand for water in California is 20 to 21 exajoules at current levels of water demand, it also must be said the fluid is high energy, and that energy will be wasted if it is merely cooled without doing work. If the high pressure is released by expanding against a turbine, additional work, exergy, can be recovered from the energy expended to perform the separation of salt and water, with the added benefit of cooling via adiabatic expansion. If, indeed, some of the heat is then used to drive a gas brayton cycle, say with CO2 as the working fluid, or alternatively, a suitable hydrocarbon or ether, even more exergy can be recovered.
I will not deny that I am a critic of the electrolytic approach to producing hydrogen, which has remained trivial for two centuries after its discovery for a reason. I often note that electricity is a thermodynamically degraded form of energy the storage of which further degrades its quality, particularly in the case where hydrogen is the storage medium. We have a lot of morons running around seeming to believe that electricity is "clean" because they spend their time worshipping stupid marketing pictures of destroyed wilderness industrialized into wind parks and deserts covered by solar trash. Right now the reality is very different. Electricity is a very dirty - and wasteful - form of energy. It is increasingly generated using dangerous fossil fuels.
When I discuss that the storage of electricity makes it even more dirty than it already is, I also add the caveat that it is acceptable to store electricity that is generated as a side product, i.e., by raising the Carnot efficiency of a system, generally via the utilization of high temperatures. Supercritical water is a high temperature fluid, and in fact, there has been a lot of work invested in developing power plants using it as a working fluid, chiefly in coal and nuclear plants. Nuclear plants are essential for the survival of our planet; it is essential to ban coal for the survival of our planet.
How the energy of supercritical water might be used in a California whose water is supplied as a desalination tool offers many varied opportunities. If the heat is provided without the generation of fossil fuel waste, large portions of it might well be diverted to produce electricity reliably and continuously, and in fact, in larger amounts that the grid requires even on its worst day, a day like that of the extreme temperatures referenced above, 06/18/21. On 06/18/21, the power demand peaked at 17:55 PDT, 5:55 PM, at 40,743 MW. At no point in the associated 24 hour period did the power demand fall below the peak demand observed on 05/01/22, 20:15 PDT, 8:15 PM, 25,016 MW (after the sun had fallen), 05/01/22 being the day which produced so much inane cheering in the media about "100% renewable" achieved at 14:35 PDT when the power demand was 15,804 MW, slightly higher than that mild day's minimum, 14,810 MW, observed at 12:25, just after noon.
Big deal. Big, big, big deal. The more we embrace the lies we tell ourselves, the worse not only for us, but for for everyone depending on the stability of the planet.
Suppose the electricity supply from expanding supercritical desalinated water against a turbine were set higher than the worst case described above, say 50,000 MW of electricity. At 35% efficiency this amounts to about 4.5 EJ of heat, a fraction of the heat available. For almost 100% of the time, this electricity would be excess, and might be diverted to other purposes. Since I have suspended my hostility to electrolysis by applying the exergy recovery qualifier, I will refer to the Willauer technology for producing jet fuel from electricity and seawater described in multiple patent applications by the US Naval laboratory as well as a number of scientific publications. Here's one:
Feasibility of CO2 Extraction from Seawater and Simultaneous Hydrogen Gas Generation Using a Novel and Robust Electrolytic Cation Exchange Module Based on Continuous Electrodeionization Technology Heather D. Willauer, Felice DiMascio, Dennis R. Hardy, and Frederick W. Williams Industrial & Engineering Chemistry Research 2014 53 (31), 12192-12200
Development of an Electrolytic Cation Exchange Module for the Simultaneous Extraction of Carbon Dioxide and Hydrogen Gas from Natural Seawater Heather D. Willauer, Felice DiMascio, Dennis R. Hardy, and Frederick W. Williams Energy & Fuels 2017 31 (2), 1723-1730
Dr. Willauer works for the US Navy. The idea motivating her work was the observation that it is expensive to transport jet fuel for fighter planes to nuclear powered aircraft carriers at sea, and so it would be wise to manufacture jet fuel at sea, using Fischer Tropsch chemistry with syngas as a starting material.
The process is said to be economical or break even at petroleum prices of around $6.00/gallon. Um, um, um...
There is no reason to make FT jet fuel from syngas. FT diesel fuel is said to be roughly comparable in environmental impact as a biofuel to other biofuels:

The caption:
Source:
Environmental, Economic, and Scalability Considerations of Selected Bio-Derived Blendstocks for Mixing-Controlled Compression Ignition Engines Andrew W. Bartling, Pahola Thathiana Benavides, Steven D. Phillips, Troy Hawkins, Avantika Singh, Matthew Wiatrowski, Eric C. D. Tan, Christopher Kinchin, Longwen Ou, Hao Cai, Mary Biddy, Ling Tao, Andrew Young, Kathleen Brown, Shuyun Li, Yunhua Zhu, Lesley J. Snowden-Swan, Chirag R. Mevawala, and Daniel J. Gaspar ACS Sustainable Chemistry & Engineering 2022 10 (20), 6699-6712
(Note that this paper assumes reforming biomass and not Willauer technology, and thus the rating in the chart for baseline carbon efficiency and target carbon efficiency under Willauer conditions would be positive, not neutral or negative.)
Other opportunities for the use of side product electricity would be for the production of metals. Magnesium metal has been isolated from seawater, depending on the cost and availability of mined magnesium minerals. Such isolation is electrolytic, and as noted above, magnesium is significant constituent of seawater. In addition, electrolytic processes are used for the manufacture of aluminum, and now, with the development of the flexible FFC Cambridge process, can be extended to many other metals, notably titanium. The isolated sodium chloride is actually far below the demand for salt in this country, and it is notable that PVC pipes, which can effectively sequester both chlorine and carbon while serving to transport water. I have also referred elsewhere in this space to the reduction of carbon dioxide to elemental carbon in electrolytic molten carbonate baths using either molten barium carbonate, strontium carbonate, or mixtures thereof.
It should be relatively clear that supercritical water desalination could actually remanufacture the entire economy of California while returning the State to livability, while also allowing all of its water to be diverted to environmental purposes. Under these conditions, it is conceivable to free the Colorado River - or at least give all of its water to Arizona and Nevada (I prefer freeing the river, but need to be realistic), restore John Muir's beloved Hetch Hetchy valley, refilling Owen's lake which was drained to fill swimming pools and water golf courses in Los Angeles, as well as making it possible to flush toilets.
But we're talking about 20 Exajoules of energy. Whence the energy?
In California history, seven commercial nuclear reactors have operated, three at San Onofre, two at Diablo Canyon (still operating but under attack), one at Rancho Seco, and one at Humbolt Bay. The quantities of slightly enriched uranium loaded into these reactors can be found here: Spent Nuclear Fuel and High-Level Radioactive Waste Inventory Report (I find the use of the term "waste" in the title to be rather dubious, nothing that is useful is "waste" or at least need not be.) By leafing through this document one can discern that the total amount of uranium initially loaded into these reactors was about 3058.13 metric tons. More than half, over 1600 metric tons is at San Onfre.
A rule of thumb for used nuclear fuel is that about 94% of it is unreacted uranium, 1% is plutonium containing small amounts of neptunium and, depending on the amount of time spent after removal from the reactor, americium and trace curium, about 5% is fission products, some of which are radioactive and some of which, depending on time, are not. It can be shown that the energy value of the actinides in the used nuclear fuel, in the case where the residual uranium is converted to plutonium is about 230 Exajoules, more than twice the annual energy demand of the entire United States, and roughly equal to the requirement for providing all of the energy demand associated with supercritical water desalination required for agricultural and urban water in California for over a decade.
I note that these calculations are purely "back of the envelope" and the infrastructure to accomplish it does not exist, although the processes for electrochemical molten salt reprocessing of used nuclear fuel is well understood. (I can certainly think of further refinements.) The cost of the infrastructure might be defrayed by selling or utilizing some of the fission products. It can be shown that the rhodium in California's used nuclear fuel is probably worth over two billion dollars alone - current prices run at about half a million dollars per kg - never mind multiple radioactive and non radioactive materials also of extremely high value. It's noting that in "breed and burn" type reactors, utilizing the fast neutron spectrum with plutonium as the seed nuclei, the yield of rhodium is roughly double than that obtained by the thermal spectrum on which California's seven reactors operated. The highest value of all, however, is neither rhodium, or palladium nor ruthenium or gamma emitters for environmental remediation; it is the energy content.
I do not live in California anymore. My wife and I divorced California nearly 3 decades ago, but we all shared many beautiful moments together there, and worn though it may be, the love, and the memory of the love, is still there. In my opinion, in the United States at least - irrespective of the extreme heat tragedy playing out in India now, and likely to play out elsewhere this summer - California is the front line of the climate disaster now well underway. I regard the official policies - and I speak as a firm Democrat - of California with respect to climate change, the notion that so called "renewable energy" will save the day, to be insipid, but there is some evidence, given recent statements by Governor Newsome and recent polling, that a sense of reality may be sinking in. The State is on the front lines of the climate crisis.
It will break my heart if California dies from climate change and the resultant lack of water.
I recognize that these musings are somewhat lengthy, convoluted and perhaps more than a little esoteric, but it is, for what it's worth, a reflection of rethinking our biases. The world can be saved, but only if we think fast and think anew.
Have a pleasant work week.
calikid
(584 posts)hunter
(38,317 posts)Two quick quips:
There's a lot of land in California that shouldn't be farmed, no matter the water source, and for a wide variety of environmental reasons. These lands ought to be restored to something resembling a natural state.
And personally, I think California should abandon the factory farm dairy industry. It uses huge amounts of water, damages the natural environment, and isn't nice for the cows. Cheap hamburger and dairy products are not human necessities.
Those measure would reduce demand for water in California considerably. For all I care the dairy farmers can move away to places where the land is irrigated by rainfall. That opinion probably makes me a traitor to my people. Blame it on my grandmother and her sister. They didn't like cows or dairymen so they fled their family business. But it's sort of weird living in a place where roads are named after my great grandparents' aunts, uncles, cousins, siblings, and other assorted relatives.
Second quip, Magnesium.
Magnesium was for a time a darling of the aerospace industry.
These uses have largely been eclipsed by sophisticated aluminum alloys.
Aluminum comes from mines.
Magnesium could be produced as a byproduct of nuclear powered supercritical desalinization.
NNadir
(33,525 posts)...I was getting tired of writing it and just wanted to put it up, as the drought is freaking me out.
I had planned to discuss quite a bit more about magnesium but I ran out of time.
It is not that magnesium has been displaced by its properties so much that it's been displaced by its cost. The world still produces just under a million tons of it, almost all of it in China from mined dolomite. Like the common metal titanium - this is about to change - and at one time aluminum, it was not the available sources that were the limitation, but rather the cost of isolating the element.
It is still an important alloying agent, particularly for preventing corrosion, about 45% of the world production is alloyed with aluminum. (My son knows more about this than I do since his Masters work was in metallurgy, but I still feel free to wax romantic on the subject.)
Overwhelmingly, the Chinese us the very dirty Pidgeon process to produce magnesium, shown graphically below:

The caption:
Source: Yang Tian, Lipeng Wang, Bin Yang, Yongnian Dai, Baoqiang Xu, Fei Wang, Neng Xiong, Comparative evaluation of energy and resource consumption for vacuum carbothermal reduction and Pidgeon process used in magnesium production, Journal of Magnesium and Alloys, Volume 10, Issue 3, 2022, Pages 697-706.
This of course, is a carbon intensive process; like hydrogen in China, magnesium production is dependent on coal.
We may compare this with electrolytic production from seawater, which under the scenario I described in the OP, where electricity is a side product sold to the grid only when needed.
Here's a nice description of the seawater approach:

Source: Valuable Metals—Recovery Processes, Current Trends, and Recycling Strategies (P. Fröhlich, T. Lorenz, G. Martin, B. Brett, M. Bertau, Angew. Chem. Int. Ed. 2017, 56, 2544.)
Reaction (17) is roasting; reaction (18) is electrolytic. I'm not quite sure that in the "thought experiment" I proposed that this would be the ideal approach, but it would work, in particular because even before getting to supercritical water desalination, I envision two higher temperature processes, either of which can be diverted to calcining. (Chlorine is a side product.)
This paper continues with a description of the electrolytic process:

Alternatively MgCl2 can be made simply by adding HCl gas to the system.
Under the conditions of my thought experiment, some abandoned processes, such as electrolytic metal preparation, and perhaps processes like the Solvay process for sodium carbonate might well prove superior to the processes that temporarily replaced them.
Recently I was reviewing some information about the FFC Cambridge process, which I think will ultimately change the world, and the point was made that it offers the opportunity to make some novel alloys that could not be prepared in the past because the boiling point of one or more of the alloying agents was higher than the melting point of another. This hadn't occurred to me, but it's certainly true. This might make a new world for magnesium alloys.
I also favor the production of carbon anodes by the electrochemical reduction of molten carbonates, about which I wrote in the past.
I certainly agree that there are large stretches of California that should be preserved. It's why I oppose the wind industry, and why I understand John Muir's losing battle to save the Hetch Hetchy valley. So called "renewable energy" is not good for the protection of wilderness. This said, the California agricultural industry is valuable to humanity. I am particularly fond of products from trees that the climate - with water - can support, avocados, almonds, cashews, citrus fruits, olives, etc. I realize that all of these have drawbacks, but speaking for myself, I value this stuff, as well as the products of the Imperial Valley, clearly a desert.
The big dream for me - I won't live to see it - is the restoration of Owen's Lake, the Hetch Hetchy Valley, the Sacramento River, stabilization of the quasi-artificial Salton Sea, stabilization of the pin oaks. (One of the most beautiful pictures I ever took of my wife was sitting on a pin oak in Pfeiffer Big Sur park. I've been pausing to look at it for some decades now.)
One of the surprises for me in researching the post besides the low concentration of carbon particulates in seawater, was the percentage of municipal trash that is actually biological in origin. It has the potential to be carbon negative in this sense.
Thanks, as always, for your comments.
hunter
(38,317 posts)... cost was not a consideration so much as weight. The same was true of high performance cars.
A magnesium engine block, for example, weighed less than the equivalent block made of aluminum.
Eventually stronger, tougher aluminum alloys were developed so that less aluminum could be used in functionally equivalent parts, which eliminated the weight advantage of magnesium parts.
As a side note, my grandfather worked with titanium for the aerospace industry when much of this technology was still secret, and much of the U.S. supply of titanium was acquired from the Soviet Union by way of shell companies run by the CIA.
Stabilizing the Salton Sea would be a very positive thing but it will require the U.S.A. deal in good faith with Mexico which will be a difficult thing so long as idiot Republicans have any power within Federal, State, and local government.
NNadir
(33,525 posts)This never occurred to me until I opened a recent review on the FFC Cambridge process, (which the Chinese are now calling the "Chen Process" after the "C" in FFC.) that discussed a topic about which I'd never thought.
The melting point of pure iron is 1,538°C. The melting point of pure titanium is 1,668°C. Nickel melts at 1455 °C.
The boiling point of magnesium is 1091 °C. It is actually low enough that magnesium can be purified by distillation, and if one were trying to make an alloy of iron and/or titanium and magnesium, the magnesium would distill away in the molten phase.
Modern alloys are very complex systems, but it has never been possible to make certain alloys because of issues in solubility and vapor pressures. With variations in over voltages, concentrations, current, one can imagine exotic materials. What, exactly would be the properties of a tungsten magnesium alloy? Who knows?
A place with lots of sea salt and cheap, reliable electricity produced by raising efficiency to unprecedented levels could run quite an FFC industry, I think, one that I note, would fairly clean.
As for the Salton Sea, I have always fantasized about supercritical water launching over the Palomar mountains into the Northern Imperial Valley in titanium pipes. It's a, um, pipe dream.
It's not like we'd like Mexico being happy, but I'd love to see the Colorado River Delta restored. (If it's allowed to erode, the Imperial Valley will ultimately fill up.) I know it's not going to happen, but it does seem remotely feasible and I think it a dream worth having.
The Colorado River is dying as we have it now, and we're both causing and letting it die. It's a huge tragedy.