Environment & Energy
Related: About this forumDefective TiO2/CdS for the Photochemical Reduction of CO2; the Capture of Radioactive Cesium.
In this post, I will discuss two seemingly unrelated papers and make some attempt to relate them to one another.
The first of these is this one: Ti3+ Defective TiO2/CdS Z-Scheme Photocatalyst for Enhancing Photocatalytic CO2 Reduction to C1–C3 Products Yunxia Bai, Mei Li, Xuemei Liu, Jinyu Han, Xinli Zhu, Qingfeng Ge, and Hua Wang Industrial & Engineering Chemistry Research 2022 61 (25), 8724-8737.
The second is this one: Adsorptive Removal of Radioactive Cesium from Model Nuclear Wastewater over Hydroxyl-Functionalized Mxene Ti3C2Tx Yujing Chi, Yuan Xu, Chenxiang Xu, Jiming Tian, Ying Li, Boxiang Gu, Haiyan Song, and Han Zhang Industrial & Engineering Chemistry Research 2022 61 (25), 9054-9066
One obvious, if trivial way the papers relate is that they both utilize the very common element titanium. Titanium is the 9th most abundant element in the Earth's crust (which is mostly oxygen and silicon) and the 7th most abundant metal if we include metals that are not really structural, such as sodium, potassium, and calcium, but the 4th most abundant structural metal after aluminum, iron, and magnesium. Titanium supplies will never be threatened. Titanium metal, as was the case with aluminum until the late 19th and early 20th century, has proved difficult to produce from the ore. The problem with aluminum was overcome with the electrochemical Hall-Heroult process relying on molten cryolite, a sodium aluminate salt; for titanium it will prove, ultimately, to be overcome by the FFC Cambridge Process, also an electrochemical process relying on molten calcium salts, assuming that the Talibans around the world, including the rogue US Supreme court featuring 6 illegitimate dishonest religious bigots, do not succeed in burdening humanity with a dictatorship of ignorant religious thugs. (Speaking of Talibans, I often muse that if the frame of the World Trade Center had been made of titanium rather than steel, they might have been damaged by the Saudi religious bigots crashing planes into them, but they'd still be standing. Titanium is lighter, stronger, and has a higher melting point than steel.)
Enough of that riff, the point is not to discuss the metals - although the Mxene is prepared using titanium metal as a starting material - but rather the chemistry of the oxidized form of titanium.
The first paper refers to photochemistry, that is chemistry driven by light. Anyone who is familiar with my writings here will recognize that I am a critic of the form of so called "renewable energy," solar energy, that gets lots and lots of cheering here and elsewhere, often on the assumption, which has quieted for a bit but has now shown up around here in abysmally stupid rhetoric, that sunlight is "free." The problem with solar energy, as I often point out, is not that massive amounts of energy arrives on the Earth - clearly it does - but that it is diffuse and variable in its availability. The diffuse property requires the unsustainable utilization of vast areas and vast amounts of material. It is the device, not the fuel that is unsustainable and prohibitively expensive. The variable property requires the unsustainable expansion of both the land area devoted to energy production, but more importantly, material, since redundancy is an inherent required feature of any system depending on the instantaneous availability of sunlight. In general processes that cannot function continuously are neither environmentally or materially sustainable. It is the variability and diffuseness that caused humanity to abandon so called "renewable energy in the 19th century, a century to which our Christian Taliban revolutionaries wants us to return in a social sense and to which allegedly liberal energy advocates want us to return in an industrial sense.
There is no justice in either of these reactionary views.
So how is it that, I, a critic of solar energy, am writing about photochemistry? I'll explain shortly. First let's look at the opening paragraph of the paper with the understanding that it contains some artifacts of translation from Chinese which explicitly refers to solar energy without reference to my criticisms above:
To date, some advances have been achieved for improving the photocatalytic CO2 reduction. Some semiconductors, such as TiO2, g-C3N4, Bi2WO6, and CdS, have been demonstrated to be capable of the formation of C2+ compounds. (16,17) Among these, TiO2 is the most widely studied one. (18−22) Excepting the architecture engineering approaches to drive the efficient CO2 reduction on TiO2, (15,17,20−22) it has been proposed that the presence of Ti3+ in TiO2 plays an important role in the formation of hydrocarbons...
So the goal of the paper is to mimic photosynthesis by using light energy to reduce carbon dioxide. One should note that photosynthesis is successful however because the catalyst is produced by self replicating systems. The systems here will not be self replicating; they need an input of energy and materials to operate. Photosynthesis relies on an organometallic catalyst; titanium is not an essential element in living systems.
Of course, the product of photosynthesis is glucose, the products here are equivalents of chemicals found in dangerous fossil fuels.
To see what these products are, let's skip ahead to one of the figures in the first paper, figure 6:

The caption:
The products are two constituents of syngas - from which pretty much any commodity chemical in either dangerous petroleum or dangerous natural gas can be made - carbon monoxide and hydrogen, as well as, from the empirical formulas, the actual constituents of dangerous natural gas, methane, ethane, ethene (aka ethylene), and propane.
Thus the point of this system is that it essentially reverses the combustion of dangerous natural gas, of course, consistent with the laws of thermodynamics, with an input of energy, in this case light energy. An important point is that this system will require more energy than the energy produced by burning the dangerous natural gas in the first place. This is what we are leaving for future generations, the requirement that to have a sustainable world they must both produce energy for their own use as well as more than all the energy we produced in destroying their world.
Quite a legacy for our generation - as they say in Canada - eh?
This system relies on a "Z-scheme" which is technical talk for placing two semiconductors in proximity to one another to optimize electron flows in the system with input of energy. In this case, the two semiconductors are titanium oxide and cadmium sulfide. (The related compound to the latter cadmium selenide is a constituent of some commercial solar cells, a fact I personally find disturbing.) The physical form of the photocatalyst is as "nanoflowers" - abbreviated "NF" in the text. From a figure in the text, the "nanoflowers" look like this under a transmission electron microscope and other nanoscale imaging devices:

The caption:
Now I'd like to get to the operative point, the nature of the "light" that inputs energy into the system to reverse the combustion of dangerous natural gas. Light, as most high school students should know, is electromagnetic radiation and the term in the scientific literature and in some cases the popular literature, makes a distinction between visible light, the colors of the rainbow, and light which cannot be seen by humans, ultraviolet light, UV, and infrared light, IR. The latter, often associated with heat, is irrelevant to this post and will not be discussed further. Ultraviolet light, depending on wavelength, is highly energetic, energetic enough to break chemical bonds, for instance the exceedingly strong carbon fluorine bonds associated with industrially generated greenhouse gases atmosphere as well as the increasingly exigent problem of PFAS (Poly/Per fluorinated alkyl substances) in water. There has been one, and only one, international treaty ever to effectively mitigate environmental destruction, the Montreal Protocol, which banned the use of certain chlorofluorocarbons because of their propensity to destroy the Earth's ozone layer, which protects human beings and all living things from UV radiation. Of course, UV light is often generated deliberately on Earth, both for technological reasons, for instance, sterilization, or to initiate chemical reactions, for analytical chemistry, and for amusement, relatively low energy UV light, "black light" was very popular among the hippies when I was a kid at various events, such as rock concerts, to make fluorescent paints glow.
UV light comes in three "flavors." UV-A is relatively low energy, characteristic of "black light," with wavelengths between 320 and 400 nm, some humans can detect deep violet light around 380 nm, the shorter more energetic UV is not visible but will cause paints to fluoresce. It's generally harmless, although it may cause eye problems with excessive exposure. A fair amount makes it to the surface of the Earth. Considerable UV-B, more energetic UV, with shorter wavelengths than UV-A also significantly penetrates the atmosphere, where it causes sunburns and, more problematic, melanoma.
The third region of the UV spectrum, UV-C, includes radiation with wavelengths between 100 and 280 nm. It is high energy just shy of x-ray energy. This wavelength is used for sterilization. Before the Earth evolved an oxygen atmosphere, terrestrial life was impossible, although it was possible in the oceans.
With this in mind, I'll post another figure from the first paper, Figure 4:

The caption:
Figure 4. UV–vis absorption spectra (a); band gaps converted from UV–vis adsorption spectra (b); and MS curves of the as-prepared samples and control samples (c–e). Band positions and the band gap energies of the as-prepared samples and the redox potentials of CO2 reduction reactions and H2O/O2 (f).
The "business" of this device requires absorption. For the nanoparticle cell described as giving the best yield TiO2/CdS0.6 as shown in figure 6, the maximal absorbance is reached at around 480 nm, which corresponds to blue light; the absorption extends well into the UV range. This suggests that for this system in particular, much of the solar spectrum would be wasted. The x-intercept of MS (Mott-Schottky) plots figure 6b, represents the potential energy induced for the reduction of CO2. (Strictly put, the Mott Schottsky equation is derived for planar, not spherical systems, but, not being an expert, I assume these values are probably a decent approximation.) These energies are given using the unit eV which can be converted using Planck's law, and the constant values of the speed of light and the unit electric charge to wavelength. For the TiO2/CdS0.6 sample having the best kinetics, this value is 2.25 eV. This converts to 450 nm, the color of which can be seen by plugging the value this tool: Wavelength the color converter. Lower energy light with a higher wavelength will have little effect. Thus much of the solar spectrum will not affect the reduction. The text suggests, without much detail, that ultimate electron donor, the oxidized species, is water, and that it is oxidized not to oxygen gas - which would generate potentially explosive gases, but rather to peroxide, O2-. There is many ways to remove peroxide from water, by precipitation. (Precipitation of peroxides has been utilized in the case of uranium and plutonium in reprocessing technology; other insoluble metal peroxides are known.) The figure also shows the electrochemical reduction potentials of the various chemical reactions. Excepting the independent production of hydroxyl radicals, which is only possible in any case for pure TiO2 nanoflowers, the highest energy requirement is for the production of carbon monoxide, -0.53V, and hydrogen, -0.42V, the two constituents of syngas.
Figure 4a shows the absorbance of the nanoparticles is strong far into the UV range, extending to the UV-A range. Thus energetic "light," largely invisible light is required; sunlight largely won't cut it.
Before turning to the second paper, I'd like to refer to an excellent Master's Thesis by (now) Dr. Kristina Yancey Spencer which carefully analyzed the status of used nuclear fuel in the United States a document to which I frequently refer. It may be downloaded for free here: Nationwide Used Fuel Inventory Analysis
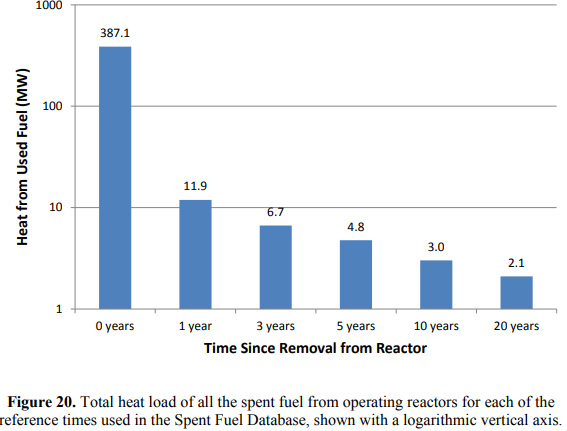
It is a comprehensive overview of all the used nuclear fuel in the United States accumulated over more than half a century without causing a significant loss of life, entirely contained, well worth a read for anyone who is, at least in my view, serious about climate change. I consider this fuel a very valuable resource that future generations may use to recover from the horror we are leaving them. The thesis gives a nice overview of both the composition and the heat load of fuels, and for the purposes of this post, I will pick out a few nice graphics from the thesis. The first of these shows the heat load of fuels.
The next graphic, parts (a) and (b) - most relevant to my point here - shows the composition of the radioactive components of the fuels that are creating the heat.


The caption for (a) and (b):
The important point is that the main radioactive isotopes providing the heat load at the "zeroth" day are not present at all in 20 years. (Note that this graphic assumes that all of the nuclear fuel were released on the same day; it is illustrative, but not a representation of the actual timing of used nuclear fuel removal from nuclear reactors. The actual removal is well staggered.) The fraction of heat being produced in the 20th year as compared to the zeroth year is 2.1/387.1 = .0054 or in the "percent talk" that is routinely used to excuse the failure of so called "renewable energy" to address climate change, 0.54% of the original heat; the ordinate in the bar graph is logarithmic.
On the other hand, within a few days of removal, all of the constituents found in the 20th year graphic, part (b), or their immediate short lived precursors were present on the zeroth day. It is the short half-lives of the constituents on the zeroth day that provides most the heat; the general rule is that the shorter the half-life of a radionuclide, the higher its specific activity and generally, the higher the specific heat.
Consider the Np-239 which provides 42% of the 387.1 MW of heat on the "zeroth" day. The half-life of Np-239 is 2.356 days. The decay energy is 677 keV. Walking through some very basic nuclear calculations, we see that the specific activity is 8.577 X 10^15 Beq per gram, and that the specific heat of this nuclide is roughly 920 Watts per gram. At 42% of the heat load, Neptunium-239 is producing 162.6 MW of continuous power. All of this power is generated by 177 kg of Np-239. After 2 weeks, 14 days, using the radioactive decay law, the fraction remaining is 0.0163, or in "percent talk" 1.63%. The power of the Np-239 decay has fallen to 2.64 MW and the mass of Np-239 to 2.87 kg. The half-life of the other major heat generating isotope, U-239, the parent of Np-239, is 23.45 minutes, so short that it is almost immediately in secular equilibrium with its daughter isotope. (Secular equilibrium in the case where no new U-239 is being produced by neutron capture in U-238 will be established in a little more than 169 minutes, but probably will have been established by the time of shutdown.) After 12 hours, 0.6 billionths of U-239 will remain, and the power output from U-239 will essentially be zero.
After 20 years, none of the isotopes producing major quantities of heat will still be present in significant quantities. The isotopes that are producing heat after 20 years were all present at the time of shutdown, the "zeroth" year, and all of them were contained in the pie slice "other" in pie chart (a).
With reference to the paper cited on the outset, I will focus on the very interesting - and in my opinion, which may not be widely held valuable - isotope Cs-137, which provides 14% of the heat load after 20 years according to Dr. Yancey-Spencer. By itself, Cs-137 is almost a pure β emitter, but it decays to a nuclear isomer, Ba-137m, of the stable nucleus Ba-137. Ba-137m decays to Ba-137 via the emission of gamma radiation, x-radiation, and Auger conversion electrons (CE) with high energy, 661.7 keV, corresponding to a wavelength of 1.87 nm, the near gamma/far x-ray range.
We can back calculate from figure 21 (b) how much Cs-137, all of it contained in the "other" pie slice at the "zeroth" year, was present then. The half-life of Cs-137 is 30.08 years. After 20 years, it is providing 15% of 2.1 MW of heat or roughly 320 kW. Again using the radioactive decay law, we see that after 20 years, the fraction of the original Cs-137 remaining is 0.631, 63.1% in "percent talk," so the power output of Cs-137 in the freshly removed nuclear fuel was roughly half a Megawatt. Now of course, nuclear fuel is removed from some reactors annually, so there is nuclear fuel that is 50 years old, and some that is only two years old, some five years old, so the inventory of Cs-137 is larger than the figure for 20 years, considerably larger. Using the figures just obtained, and integrating the radioactive decay law from zero to 20, we can estimate that the power output of all the Cs-137 collected over 20 years as being on the order of 30 MW, much of it present as gamma radiation from the isomeric transition of Ba-137m to Ba-137, for which Dr. Yancey Spencer has surely accounted in her excellent Master's thesis.
For a number of years, for various reasons, I've spent a fair amount of time thinking about the properties of barium fluoride, one of which is its use as a scintillating crystal for the down conversion of gamma radiation to UV radiation. I must have read it somewhere, but since I was relying on memory, I downloaded, arbitrarily, a paper to confirm that my memory is accurate:
W Kononenko, J.G Heinrich, N.S Lockyer, J.M Miller, C Woody, S Kwan, Photon emission from 511 keV gamma rays incident on BaF2 and LaF3:Nd3+ crystals using a cesium iodide photocathode detector, Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, Volume 396, Issues 1–2.
The accumulation of Cs-137 in used nuclear fuel is controlled by the Bateman equation in the form utilized to model the coupled evolution of quantities of fission products in the presence of a neutron flux. An operative form of this equation that makes it easy to visualize the situation during the use of nuclear fuel is this:
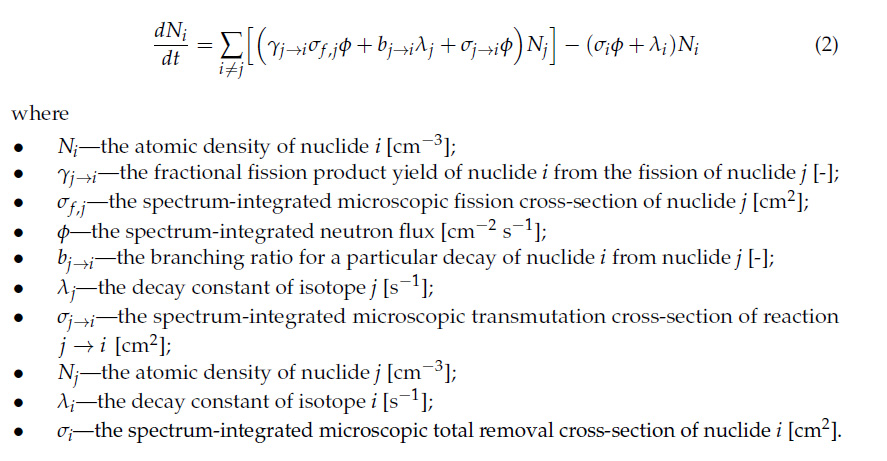
The neutron capture cross section, σj, of Cs-137 is trivial - it cannot practically be transmuted into Ba-138 - and of course, the fuel is in the reactor for only three years on average. Once the fuel is removed from the reactor, the value of the flux, φ, is essentially zero except for trivial spontaneous fission events in the actinides, and similarly the fission yield, γ i→j is also essentially zero. It can be shown that the above equation under these conditions with appropriate approximations can be reduced to a differential equation whose solution is of the form N(t) = P(1 - exp(-λt)), where P is a function of the power level of nuclear energy output, essentially the maximum amount of Cs-137 that can accumulate before it is decaying as rapidly as it is formed, where the new quantities accumulate is essentially zero. This equation shows that this value is approached asymptotically at a constant power level.
Because of the triumph of fear and ignorance - the signature mentality of anti-nukes who run around saying that nuclear energy and only nuclear energy is "too dangerous," and thus that vast death toll of air pollution and heat stroke from climate change is not "too dangerous," the growth of nuclear energy on this planet has been, to the detriment of every living thing on this planet has been arrested. The power level on the entire planet has hovered just below 30 Exajoules/year since the early 1990's without any growth. I hear people I detest cheering for this outcome.
I have in my files a spreadsheet that uses this approximation to show how much Cs-137 will accumulate at any power level at any particular year. For a power level of 30EJ/year, this approximate value of accumulated Cs-137 is 242 MT for all of the nuclear energy produced on this entire planet in the last 22 years of this century.
In the last 22 years the amount of the dangerous fossil fuel waste carbon dioxide dumped into the planetary atmosphere at a rate approximating 35 billion tons per year - and rising - is somewhere around 750 - 800 billion tons, accumulated while we all waited insipidly for the solar and wind nirvana which has cost well over 3 trillion dollars in this century and has only recently managed to produce 10.4 EJ of energy in a single year, about 1/3 of the energy that nuclear energy has been producing for 30 years in a climate of vicious and ignorant hostility and vituperation. The expensive dream of this solar and wind nirvana has done nothing, zilch, nada, zero to address climate change. In July of 2000, the concentration of the dangerous fossil fuel waste in the planetary atmosphere - never mind the amount absorbed into the seas acidifying them - has risen from 370.13 ppm recorded in the week beginning July 9, 2000, to 421.13 ppm reported at the Mauna Loa Observatory last week, an increase of over 50 ppm, in this century.
Yeah, solar and wind will save us. Right. Sure. Let's bet the future of humanity on that concept despite all of the experimental evidence to the contrary that is hasn’t worked, isn’t working and won’t work.
History will not forgive us, nor should it.
We live in an age dominated by a waste mentality. We make lots of noise about recycling our consumer "stuff," but as a practical matter all efforts to do so are trivial, because recycling matter requires energy, copious energy, and because we remain wholly and totally dependent on the mining and combustion of dangerous fossil fuels for energy and the free unrestrained dumping of dangerous fossil fuel waste on a scale of hundreds of billions of tons each decade this is self-defeating.
Somehow, with selective attention and downright stupidity the only putative "waste" to which people react whenever energy technologies are discussed is so called "nuclear waste." The idea to dispose of this putative "waste" is to bury the stuff, an idea applauded, I might add, by many people who hold similar views to my view that nuclear energy is the last, best hope of the human race.
I dissent. I object to the concept of "waste," and in doing so, I am fully aware of the energy requirements that eliminating "waste" entails.
Above, in citing the paper on the photochemical reduction of carbon dioxide with "Z-scheme" nanoparticles, I have implied that the system might run on gamma radiation down converted to UV radiation in a constant and reliable source of electromagnetic radiation provided by a radioactive material like Cs-137. This almost certainly won't happen with the nanoparticles so carefully prepared and studied by the fine scientists who wrote the paper, who were, in any case, misleading in stating that this was a solar technology. It's OK by me; they have to get funding grants, and money often flows from popular fantasy.
This paper just explored is one of many thousands of papers on the photochemical reduction of CO2 published each year, along with many thousands on the electrochemical reduction of CO2, along with many thousands on the reductive hydrogenation of CO2. Perhaps an international coalition might pick the best of these and industrialize a few, but this won't happen. In my opinion, we'll just continue to lie to ourselves, until the last molecule of CO2 finds its way into the sea or the atmosphere.
I have uses in mind for Cs-137, which I regard as a wonderful nuclide, uses involving "recharging" the radioactive of fission product cesium which includes the direct production of three isotopes, the non-radioactive Cs-133 isotope, the long lived Cs-135 isotope, and Cs-137 in roughly a 2:1:2 ratio respectively, and the production of a fourth isotope, Cs-134, indirectly formed from the non-radioactive Cs-133 as a consequence of the Bateman equation above.
One use, about which I'll say very little more, involves it's dissolution in aqueous solutions which I propose to expose directly to the environment. In this case, it would be wise to be able to remove trace amounts of the isotope, recovering it for further use in the process.
This brings me to the second paper, the one entitled Adsorptive Removal of Radioactive Cesium from Model Nuclear Wastewater over Hydroxyl-Functionalized Mxene Ti3C2Tx.
Mxenes are layered materials generated by the treatment of "MAX Phases" the development of which, albeit not the discovery, was driven by the remarkable work of the Egyptian American materials scientist Marcel Barsoum of Drexel University. Pretty much every paper now written about MAX Phases and/or Mxenes cites him.
Now let's turn to that fine paper, despite artifacts of the translation from Chinese, the introduction of which contains a number of misleading statements as well as some accurate ones. That introduction:
True statements:
The yield of 137Cs is (in thermal U235 powered reactors that dominate the world nuclear fleet) around 6.10%.
The half-life given, 30.2 years varies from the figure at BNL (30.2 Y vs 30.08 y), but there is some variance among the measurements, I have chosen the ENDF measurements above; this difference is trivial.
The elements listed as components of used nuclear fuel fission products and reference to the actinides, including details not stated, that is that they neptunium, plutonium, americium and traces of curium, are more or less correct, but it must be noted that slightly less than 95% of used nuclear fuel in general is simply unreacted uranium.
137Cs, as noted above, does emit gamma rays, it is water soluble, and it does, when it's been released in nuclear weapons testing and in events like the big boogeymen at Chernobyl and Fukushima, it is known to complex with environmental matrices. (It should however be noted that the effect of this complexation, can be to reduce bioavailability for absorption into biological matrices including, but not limited to human flesh. cf, for example, Sang-Min Park, Daniel S. Alessi, Kitae Baek, Selective adsorption and irreversible fixation behavior of cesium onto 2:1 layered clay mineral: A mini review, Journal of Hazardous Materials, Volume 369, 2019, Pages 569-576.
The bioavailability half-life of cesium isotopes is far lower than the decay half-life.)
It is also true that in vivo cesium behaves very much like sodium and potassium, more like the latter than the former, but it is also true that as a result, since potassium and sodium are released in urine, that this greatly reduces the biological half-life of cesium in flesh, which in any case, is very unlikely to be in concentrations higher than the natural levels of radioactive potassium-40, about 4000 Beq in a normal 70 kg human being. Having no radioactive potassium in one's body would be fatal, as potassium is an essential element.
It is also true that the chief means of removing cesium from water is adsorption, generally on to ion exchange resins, and more rarely into gels or porous solid phase extraction agents or metallo organic frameworks (MOFs). The proposal to use Mxenes is, to my less than comprehensive knowledge, new.
Now the misleading statements:
The question of whether cesium is "dangerous" can be misleading as well. It is true that a few people have been killed in accidents involving cesium-137, notably the accident at Goiânia, Brazil, where a radiotherapy medical instrument containing 137Cs was abandoned and disassembled by barely literate scavengers. It is likely that the fire fighters at Chernobyl who flew into clouds of smoke to drop borated sand on the flaming reactor were probably killed by volatile cesium isotopes, along with those of other volatile elements, notably iodine-131. A great hullabaloo was raised about cesium releases into the ocean after the Fukushima event, but there is very little evidence that these releases killed as many people, or, in fact, any people as did the coal waste released to power computers to carry on about Fukushima endlessly on the internet while air pollution kills seven million people per year. I contend that properly handled, using existing technology or perhaps improved technology which this Mxene may well represent, 137Cs can be used to save lives, and I'm not just referring to radiotherapy for cancer. Gamma rays can address some very serious environmental problems.
Finally it is extremely misleading to imply that cesium and other so called "nuclear wastes" are released into the environment on a ton scale where they represent a huge environmental and safety problem. The discussion of "highly radioactive wastewater" flowing into the ocean is nonsense, except perhaps in the case of Fukushima, but there is very little evidence that the flows at Fukushima have represented a huge health risk other than the coal burned to power websites to carry on about them. It is true that the nuclear fuel reprocessing sites at Sellafield in the UK, and at La Hague in France, released very dilute solutions of cesium into the ocean, after ion exchange, but every gram of used nuclear fuel processed at La Hague or Sellafield saved lives; there is no evidence of a death toll from these trivial releases of cesium by these plants. La Hague and Sellafield both relied on the PUREX and related liquid extraction procedures to recover plutonium; I believe these processes, while over all excellent, should be retired because better options exist.
Let's move on. The paper continues:
References 23 and 25 are Barsoum papers.
A few pictures from the text illustrating the nature and performance of the Mxene for Cs+ removal:

The caption:

The caption:
The system is fairly selective for the group 1 ions:

The caption:
Comparison of the -OH functionalized Mxene with various other Mxenes:

The caption:
Some modeling information.

The caption:
Adsorption models

The caption:
A scheme offering a mechanism of how the -OH functionalized Mxene works:

The caption:
The authors propose these Mxenes as reusable complexing agents. I would like to suggest that a gamma emitter in the presence of titanium oxide species might prove a very valuable component for the destruction of otherwise intractable pollutants. I don't actually tremendously favor aqueous extraction techniques for used nuclear fuels, but I can certainly see the deliberate creation of them to meet particularly exigent situations in the environment, of which there are many, particularly of a chemical nature, including but hardly limited to compounds with carbon fluorine bonds.
This work is interesting, and I fully understand why the authors needed to make some general misleading statements to justify their funding. Whether it will ever prove an industrial technology, like all such papers, is a long shot.
People often speculate in interactions with me as to whether nuclear power can be made "safer." My response is uniformly, "safer than what?" Nuclear energy is not risk free. However it doesn't need to be risk free to be vastly superior to everything else, including endless and frankly destructive wishful thinking about so called "renewable energy" which has proved nothing more than faded lipstick on the extremely dangerous and clearly deadly fossil fuel pig. Wind and solar are not sustainable at a level that will allow for economic justice for the billions of people now denied it, far out of the purview of the bourgeoise who want to talk about their electric cars. In fact they are not sustainable under any circumstances. The mass and land requirements, in addition to the environmentally destructive lack of reliability preclude them from being so.
Now can nuclear energy be "safer?" It depends on how much effort and money wants to sink into it, but the returns will be diminishing if only because at this point, as few lives are at risk because of nuclear technology, there are many, many, many, many more technologies that can save vastly larger number of lives than the few that might be saved with massive and frankly, unjustified, expenditures. Should we spend a billion dollars to save one paranoid suburban idiot from worrying about a few 137Cs atoms in his bloodstream or in his tuna fish sandwich, when we could spend the same billion to construct safe sanitation systems for the more than one billion people who lack them?
This is a moral question that almost never gets asked.
In any case, I hope I've established how these two papers are related, at least in my mind.
Have a nice day and a lovely, lovely weekend.