Environment & Energy
Related: About this forumChecking the "integrity" of biofuels by the radioactive signature of carbon-14.
The paper I'll discuss in this post is this one: Decolorization of Biofuels and Biofuel Blends for Biogenic Carbon Quantification with Liquid Scintillation Radiocarbon Direct Measurement James E. Lee, Zheng-Hua Li, Earl D. Christensen, and Teresa L. Alleman Energy & Fuels 2022 36 (14), 7592-7598.
I am, in general, an opponent of so called "renewable energy" based on the onerous material and land intensity which are both environmentally destructive and unsustainable, as well as - probably most importantly - the fact that their use depends on access to dangerous fossil fuels, often expressed as a need for economically (and environmentally) questionable redundancy.
The four major forms of so called "renewable energy" can be ranked here in the order of scale of number of Exajoules of energy produced as reported in the 2021 World Energy Outlook. (The 2022 edition should come out in the next few weeks.)
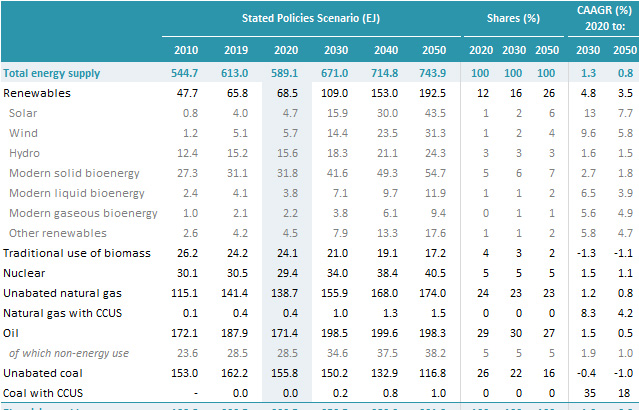
Source: IEA World Energy Outlook, 2021, page 294, Table A1A
"Modern solid phase bioenergy" is basically burning wood, as in the detestable Drax powerplant in Great Britain, where they get "renewable energy" credits for ripping up American forests with specious claims that would not stand up to a serious audit.
This is followed by wind energy, which I oppose, solar energy which I oppose except for special cases (like road signs or remote outhouses), and finally liquid biofuels, which I generally oppose as practiced now, although I am in favor of biomass pyrolysis where the heat source is nuclear and the biomass is a side product, for instance corn stover, leaf litter or yard waste. (I absolutely and emphatically oppose tearing rain forests to pieces to make palm oil plantations.)
This paper is roughly about that case, the stuff made by tearing rain forests to pieces to make palm oil plantations, and some related stuff, like diverting food supplies to run trucks. It's about biodiesel.
First, a little about carbon-14: The chief source of carbon-14, a radioactive isotope is the following nuclear reaction, written thus 14N(n,p)14C. A neutron collides with the most common stable isotope of nitrogen, the isotope nitrogen-14; resulting in the spallation of a proton from the N-14 of the nucleus to give carbon 14. This reaction takes place in the upper atmosphere, where neutrons are produced as a result of interactions of atmospheric atoms with cosmic rays, and it accounts for the ability to use carbon-14 dating before the 14C steady state was disturbed by nuclear weapons testing and, in a different direction, by the combustion of dangerous fossil fuels releasing 14C depleted ancient carbon. A smaller positive effect than these two has been used nuclear fuel reprocessing, an activity I enthusiastically support.
A very nice, straightforward and honest description of environmental 14C can be found (in English) on a webpage produced by a French Nuclear Safety Institute (ISRN) here: Carbon-14 and the environment. I'm sure the information on this fact sheet will excite the fetid imaginations of people who fear radioactive atoms more than they do the death of seven million people per year from endemic and inescapable air pollution as well as climate change, which is killing the entire planet while they whine about, say, Fukushima.
The ISRN website features this nice graphic delineating the anthropic disruption of the 14C equilibrium established over the history of the Earth:

In any case, all photosynthesis on this planet has always incorporated 14C, even before the long established atmospheric equilibrium derived from cosmic rays was disturbed by human activity, the which accounts for the ability to use carbon dating because the half life of carbon 14 is long enough to tightly resolve dates extending over the millennia of human activity, but short enough to offer fairly high precision and accuracy for such dating.
As is the case with the wind and solar industry, the biofuel industry is designed around access to dangerous fossil fuels and the release of dangerous fossil fuel waste. The world standard approach for measuring the origins of a carbon source, whether it is of recent biological origin or fossil origins by measuring the 14C content is accelerator mass spectrometry (AMS), but these instruments are expensive and getting time on them is difficult. It would be ideal to use simpler cheaper devices to get a level of honest evaluation of whether biofuels are or are not what they say they are. Hence this paper. It evaluates refining the use of instruments that rely on the light generated by nuclear decay of carbon 14 when it decays back to nitrogen-14 via beta emission.
From the introduction:
.
Although not an ASTM accepted method, direct analysis of fuels with liquid scintillation counters (LSCs) has been shown to be precise and accurate in quantifying %CBio. (4,5) Direct analysis simply involves dispensing the sample into a vial and adding the scintillation cocktail for preparation for counting. This approach is more accessible but has two limitations. First, the direct analysis approach requires two separate measurements to determine (1) the amount of 14C in the sample and (2) the total carbon mass of the sample. (5) Second, the detection efficiency of the LSC is reduced if the sample is not colorless, as is common with biofuels, and must be determined either through empirical parametrizations or through an internal spike process. Scintillation based spectroscopy depends on converting energy from decay radiation (beta particles) to light. Photons are produced through a series of reactions which multiply emissions and shift electromagnetic wavelength, primarily in the ultraviolet (UV) range. If the sample material can absorb energy at these wavelengths, then it will inhibit detection of decay events by the LSC photomultiplier tubes. This effect is called chemical quench and color quench, depending on which stage of reactions is disrupted.
To reduce the negative effects of quench, ASTM D6866-20 (3) Method C instructs users to convert the sample fuel to benzene; a material translucent to the emission energy spectra of the scintillate cocktail. As mentioned, this is a time-consuming, laborious, and potentially hazardous process. Alternative approaches include diluting the sample in an optically clear liquid (e.g., toluene) (5) and fuel conversion to CO2. (6−8) Diluting the fuel will reduce the color intensity but also reduces the sample size of biogenic carbon which negatively affects the precision of 14C determination. Conversion of the fuel to CO2 (followed by absorption of that CO2 into an amine solution) also has a limited sample size (1.6–2.5 g CO2), and the precision of this technique is currently not adequate for low blend-level fuels. (9,10)
In this study, we explored another approach, decolorization of the fuel. Decolorization is the process of removing or degrading color-causing molecules or sections of molecules, i.e., chromophores. In organic molecules, color is often related to conjugated π-bonds in benzene-ring structures such as occurs in aromatics which are a common component in petroleum fuels. Colored components can also be naturally occurring in fats and oils as antioxidants. Oils derived from lignocellulosic biomass (bio-oils or pyrolysis oils) can also have very dark colors...
The authors used two methods for decolorization of the biofuels, whether cut with dangerous fossil fuels or neat.
One was passing the biofuel mixtures or neat samples through silica gel or activated carbon. The other relied on the fact that the color of these organic species depends largely on the presence of double bonds, which can be oxidized by various means, for example ozonolysis, benzoyl peroxide or photochemistry using photocatalysts such as copper or silver for visible light, or by titanium oxide for UV light.
The authors chose photooxidation.
The pictures tell the story:

The caption:

The caption:

The caption:
Some tables from the paper follow.
Improvements in counting efficiency:

Comparison of accuracy and precision:

GCVUV-Fame uses gas chromatography and does not depend on carbon-14.
Some commentary on the results:
These analyses show that these fuels contained 2%, 22%, and 92% biogenic carbon for diesel, B20, and B100, respectively. The standard specification for diesel fuel in the US allows for up to 5% biodiesel, (42) and it is common for fuel producers to add a small quantity of biodiesel. The measured blend level of the B20 sample is higher than the stated blend level but is not outside of the observed variance. (43) The result for the B100 sample being lower than 100% is partially related to the use of methanol in the production of fatty acid esters as previously discussed. The mass percent of this nonrenewable carbon accounts for roughly 5 wt % on average (21,39) which does not fully account for the low biogenic carbon.
In the B100 sample, which is nominally 100% biodiesel, the decolored sample was found to contain the same 14C content as the unmodified sample. This observation implies that the removal of color-inducing chemical species did not affect the inferred biogenic fraction because the removed species contained the same 14C content as the bulk biodiesel fuel.
However, both the diesel and B20 samples were likely blended at the terminal, prior to being offered for sale at the commercial station. In these samples, we saw a large decrease in %CBio content of 1 and 6% for diesel and B20, respectively. While decolorization of these samples drastically reduced color intensity, we also observe a significant removal of biogenic carbon in both the reduction in 14C content and FAME. This implies that biogenic molecules are preferentially adsorbed relative to fossil hydrocarbon diesel. Chromophores are expected to account for a small fraction of the fuel composition, less than the inferred decrease in biogenic content. It is not clear whether chromophores are preferentially removed in comparison to other biogenic molecules or whether there could be an optimal quantity of adsorbent to reduce bias because fuels will vary in blend levels and in composition of the biogenic fuel.
Conclusions from the paper:
Again, I don't really support biofuels as the industry is now constructed. It's been an environmental tragedy, and this goes for ethanol as well. I do concede that because of the ability of living things to self-replicate there is some hope for utilizing some biological materials - algae seems the best to me with access to (potentially waste) heat to dewater it - via pyrolysis, but we're nowhere near that.
I trust you're having a pleasant weekend.