Environment & Energy
Related: About this forumElevated Radium Activity in a Hydrocarbon-Contaminated Aquifer
The paper I'll discuss in this post is this one: Elevated Radium Activity in a Hydrocarbon-Contaminated Aquifer, Amy K. Wiersma, Glen Hook, Madeleine Mathews, Sean R. Scott, Jessica R. Meyer, Beth L. Parker, and Matthew Ginder-Vogel Environmental Science & Technology 2023 57 (24), 8983-8993.
Before breaking in commentary, allow me to produce the synopsis of the paper:
This study reports geochemical conditions resulting in elevated naturally occurring radium in groundwater near the source zone of an organic chemical mixture, with implications for human and ecosystem health if extensively mobilized downgradient to aquatic systems and drinking water sources.
The word "synopsis" is in bold and larger type in the original; in the copied text here I have added the bold for "naturally occurring radium."
The hydrocarbon contamination described in the text is anthropogenic as the text I will cite will show, and in this case comes from releases from a chemical plant. However the largest instances, to be sure, of aquifers contaminated with hydrocarbons are those involved with fracking. The Marcellus shale, now part of a huge fracking exercise and not all that far from where I live, is notable for the large amount of "produced water" dumped on the surface. As this shale is both a geological formation of dangerous natural gas, and a uranium formation, the produced water is radioactive because the radium in the natural decay series of uranium, is extracted along with the gas into the water, brought to the surface and dumped.
There are areas where the lakes of produced water are far more radioactive than the seas around Fukushima, Fukushima being the big bogeyman that raises enthusiasm for dangerous natural gas, coal and oil, the difference between the release of radioactivity at Fukushima and the wastes of dangerous natural gas, coal and oil kill people and Fukushima on a scale of millions of people per year, and it not clear if radiation releases at Fukushima has killed anyone, although it is thought that the fear of radiation killed people.
Recently we've had hydrogen salespeople here who nonetheless carried on - stupidly in my opinion - about Fukushima, while advocating for the increased use of fossil fuels, although their industry advertising for the hydrogen industry doesn't represent what it is as such. Nevertheless, 48% of the world's hydrogen is produced by the steam reforming of dangerous natural gas, 30% by the steam reforming of petroleum, and 18% by the steam reforming of coal, the latter mostly in China, the largest consumer of coal in the world. The remaining 4% of the world's hydrogen is produced by electrolysis, mostly as a side product of the chlorine industry, which until recently, represented a major source of mercury release owing to the use of mercury anodes. (Mercury anodes have been largely displaced in the industry, but are still used in some facilities.)
Source: Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review Laura Santamaria, Gartzen Lopez, Enara Fernandez, Maria Cortazar, Aitor Arregi, Martin Olazar, and Javier Bilbao, Energy & Fuels 2021 35 (21), 17051-17084].
The salespeople in the ads run here would like you to believe that the hydrogen produced by electrolysis is "green hydrogen" produced by using electricity from the generally useless solar and wind industry, and they are always running ads featuring "green hydrogen" facilities that they say run on solar and wind. However, it is very clear that it is highly unlikely that these trivial Potemkin plants shut down whenever the wind isn't blowing and the sun isn't shining, because hysteresis, a lag time during which a current must be applied to an electrolysis device before hydrogen is produced, further degrading the already degraded economics and environmental impact of electrolysis of water. No one will audit the "green hydrogen" plants to see if the electricity really stops being applied to the electrolysis units on dark windless nights, and if they do, be sure you will see cheap accounting tricks like "offsets" that are used to greenwash the use of dangerous fossil fuels. Overall, almost all of hydrogen produced for electrolysis on this planet, the 4% in "percent talk" results from the use of dangerous fossil fuels, at a larger thermodynamic loss than reforming except, perhaps, in places like that off shore oil and gas drilling hellhole Norway.
Steam reforming of dangerous fossil fuels is not only cheaper than electrolysis, it is probably cleaner, where the bold "er" refers to the fact that "cleaner" is hardly ever the same as "clean."
So the hydrogen sales people are really in a "bait and switch" advertising campaign for dangerous fossil fuels, and are only concerned about "radioactivity" when it is present in technologies for the elimination of dangerous fossil fuels. They couldn't care less about radioactivity from the stuff they actually promote in their shell game, dangerous fossil fuels. Let's be clear, anyone promoting hydrogen is promoting dangerous fossil fuels. Soothsaying about some magical wind and solar nirvana that did not come, is not here, and will not come is not the same as actually relying on a product that requires fossil fuels in reality, and even worse, one that wastes energy for the purpose of energy storage. Hydrogen is generally not produced by electrolysis, and when it is, it is very unlikely that the electricity comes from the trivial solar and wind industries.
So then, lets turn the extraction of radium, including both chemical and biological mobilization and transport from the presence of hydrocarbons, in this case from spills and deliberate discharges.
From the paper's introduction:
Consumption of Ra over extended periods is linked to an elevated risk of bone cancer (23,24) and is therefore regulated in drinking water by the U.S. Environmental Protection Agency (EPA) at a maximum contaminant level (MCL) of 185 millibecquerel per liter (mBq/L), or five picocuries per liter (pCi/L), for the combined total of 226Ra and 228Ra. An alkaline earth metal, Ra primarily exists as a divalent cation (Ra2+) under environmentally relevant conditions. (25) Ra is produced within the uranium-238 (238U) and thorium-232 (232Th) decay series, with 226Ra produced along the 238U decay chain and 228Ra produced directly from the alpha decay of 232Th. Therefore, the distribution of parent nuclides 238U and 232Th are important considerations for Ra occurrence in groundwater. Ultimately, Ra activity (concentration) in groundwater is controlled by sorption to Fe and Mn (hydr)oxide and clay minerals, and co-precipitation with sulfate and carbonate minerals. (26−29)
Geochemical conditions that limit sorption, including elevated total dissolved solids (TDS), low pH, and reducing conditions, can result in elevated Ra in groundwater. (30) While these conditions can occur naturally, anthropogenic activities can also alter aquifer geochemical conditions and subsequently influence Ra occurrence in groundwater. For example, increased TDS due to road salt application results in competition for sorption sites and the prevalence of mobile Ra-chloride complexes, increasing Ra activities in groundwater over decadal timescales. (31−33) Seawater intrusion can also increase aquifer TDS, subsequently mobilizing Ra. (34,35) The infiltration of brines from oil production into groundwater can alter salinity, pH, and redox conditions, releasing Ra from aquifer sediments to groundwater. (36,37)
The spill of organic chemicals that reaches the subsurface is another example of anthropogenic influence on aquifer geochemical conditions that could potentially impact Ra occurrence in groundwater. This study evaluates Ra (226Ra + 228Ra) and parent nuclide (e.g., 238U) occurrence in a sandstone aquifer contaminated with a mixture of chlorinated solvents, ketones, and aromatics occurring as a dense non-aqueous phase liquid (DNAPL) in the source zone. The objectives of this study are to (1) compare Ra occurrence within the influence of contamination relative to a background reference location, (2) identify geochemical conditions associated with elevated Ra activity within the influence of contamination, and (3) evaluate potential Ra attenuation mechanisms within the dissolved phase contaminant plume. A network of high-resolution multi-level monitoring systems is used to compare Ra activities in groundwater at three locations: (1) directly downgradient, but not in the DNAPL source zone, (2) near the middle of the dissolved phase hydrocarbon plume, and (3) a background location outside the influence of hydrocarbon contamination. (38) Measured aqueous parameters used to assess 226Ra activities in relation to geochemical conditions induced by organic contaminant occurrence and biodegradation include redox parameters (e.g., nitrate, sulfate, Fe, Mn), TDS, and pH. Geochemical modeling is applied to evaluate Ra sequestration mechanisms within the dissolved phase plume (e.g., co-precipitation, sorption). Overall, this study assesses the potential for the release of naturally occurring Ra from aquifer sediments at hydrocarbon-impacted sites and highlights the importance of characterizing trace elements to achieve a holistic assessment of the potential health and environmental impacts at such sites...
Some graphics from the text:
The cartoon for the abstract:

The geographical location:
The caption:

The caption:

The caption:

The caption:

The caption:

The caption:
From the paper's conclusion:
The authors go on to discuss some sites in Cape Cod and in New Jersey that might be investigated for similar effects.
What is to be done about radium? Actually not much can be done other than to prevent its mobilization by anthropogenic activities. Uranium, the parent of radium, is a natural product, of course, widely distributed in Earth's crust. Radium, which has a half-life of around 1610 years is in secular equilibrium with its parent compound, decaying as rapidly as it is formed, with the ratio at this equilibrium, when undisturbed, existing in the ratio of its activity constants, these being themselves determined by dividing the natural logarithm of 2 by the half life in equivalent time units. For example, Ra-226, the radioactive daughter in the decay series of Uranium-238 with a half life of 4.5 billion years is at equilibrium (ln(2)/4,500,000,000)/ln(2)/1610) = 1610/4,500,000,000 = 3.58 X 10^(-7). Economically recoverable terrestrial ores of uranium at current prices are thought to be around 6,000,000 tons, suggesting that there are about 2 tons of radium in them at secular equilibrium. However economically recoverable ores are only a small fraction of the uranium found in the geochemical uranium cycle which involves the uplift of mantle rocks, where the decay of uranium accounts for much of the internal heat of the Earth, cycling through the crust on geological time scales, weathering of crustal rocks by rivers, glaciers and the like, followed by flow ultimately into the oceans where uranium is present on the scale of 4.5 billion tons.
(cf. S. Krishnaswami and J. Kirk Cochrane, eds. U-Th Nuclides in Aquatic Systems. Vol 13 of the Radioactivity in the Environment Series, U and Th-Series Nuclides as Tracers of Particle Dynamics, Scavenging and Biogeochemical cycles, Elsevier, 2008.)
Recoverable uranium ores represent only a trivial portion of the uranium in the continental crust. The mass of the continental crust is taken to be around 2.2 X 10^(24) kg, or 2.2 X 10^21 tons.
Source: Seema Kumari, Debajyoti Paul, Andreas Stracke, Constraints on Archean crust formation from open system models of Earth evolution, Chemical Geology, Volume 530, 2019, 119307
Uranium is thought to represent about 2.75 ppm of this mass, roughly as common in Earth's crust as tin.
Source: Herring, Uranium and Thorium Resources Encyclopedia of Sustainability Science and Technology (2012)
It follows that crustal rock contains over 6 trillion tons of uranium, suggesting that a secular equilibrium if unextracted by chemical means, that it also contains about 22 million tons of radium.
There is obviously a considerable amount of radium that in theory could be extracted by chemical means, including from chemical and dangerous fossil fuel extraction procedures, now endorsed, albeit with disingenuous "bait and switch" tactics using the useless solar and wind industry as a marketing tool to say that what is happening isn't happening - literally gaslighting, since hydrogen is mostly made from dangerous natural gas, by the "hydrogen will save us" industry, pure thermodynamic nonsense.
Most of this radium will not be extracted of course, but we mess with Earth's crust at our peril. Extracting even a small fraction of 22 million tons is problematic. All this said, uranium itself is extracted by water weathering of crustal rock and it does end up in the oceans, as mentioned above, and a great deal of work has been done to show the economics and feasibility of extracting uranium from seawater, although at this time, terrestrial ores are much cheaper, if less environmentally benign.
I discussed this at length some years back on another website, maintained by Professor Barry Brook, an Australian Academic, to strongly suggest that uranium is inexhaustible: Is Uranium Exhaustible?.
One thing that might be done, and this may be feasible at the Marcellus shale after the gas is gone, assuming the planet survives the use of dangerous fossil fuels while we all wait stupidly and increasingly breathlessly for the grand so called "renewable energy" nirvana that has not come, is not here and won't come, would be extract and destroy the uranium parent, recovering vast amounts of clean energy in the process. A new process for uranium mining, related to fracking but less noxious, relies on extraction procedures of this type, and as the Marcellus shale is already destroyed for the rest of the history of humanity, these techniques could be switched, perhaps using supercritical carbon dioxide, to both chemically clean the crustal industrial mess while recovering uranium for use.
About four or five years ago a new type of antinuke began showing up at DU to add to the traditional antinukes who have always argued that nuclear energy is "too expensive," "too dangerous," "too this," "too that," blah, blah, blah which, by extension, as a practical matter, is the same as arguing that "climate change is not too dangerous," "fossil fuels are not too dangerous," "ocean acidification is not too dangerous" and so on. This is the "I'm not an antinuke" antinuke, a breed that shows up with increasing frequency, a set of poorly educated and poor thinking individuals who concede that while nuclear energy is effective against climate change, it's, um, well, "too expensive," "too dangerous," "too this," "too that," blah, blah, blah, advancing every moronic specious argument against nuclear energy, including the extremely disingenuous "problem" of so called "nuclear waste," despite the spectacular record of used nuclear fuel in not killing anyone.
This goes on while the world burns and people all over the world die from extreme heat, never mind air pollution.
One of the first "I'm not an antinuke" antinukes I ever encountered at DU was a very weak thinking person who called up one of my old posts to tell me that a tunnel had collapsed at the Hanford Nuclear Weapons site and thus, by implication, that we should continue to let 7 million people die each year from air pollution, because this particular shit-for-brains assumed that the release of any radioactive materials anywhere was an international tragedy, a bit of absurd nonsense that is carried on to this day by illiterate journalists from our "but her emails" media, and of course, straight up antinukes and "I'm not an antinuke" antinukes.
I mocked this particular idiot by suggesting that his terror that a radioactive atom might show up in his, her, or their tiny brain - it would die without potassium, which is naturally radioactive in his, her, or their brain, which led to some very stupid outrage on the part of this person, carrying on in response to sarcasm - antinukes are generally witless - about "straw men."
This particular bit of nonsense actually turned out to be inspiring, and I'm grateful for it, as it caused to wonder exactly how many atoms would end up in an antinuke's tiny brain from, say, a tunnel collapse at Hanford.
I learned a tremendous amount while looking into this matter, which took on a source of intellectual inquiry of its own, to write this long, lugubrious post: 828 Underground Nuclear Tests, Plutonium Migration in Nevada, Dunning, Kruger, Strawmen, and Tunnels
In that post, I referenced a work in German, showing that in the case of actinide recycling to recover the many valuable materials in used nuclear fuel, and to extract the maximal energy from it, that after a few hundred years, the radioactivity associated with uranium ores would be reduced because its multiple radioactive daughters would not be formed:
The following figure shows the very different case obtained if one separates the uranium, plutonium and minor actinides (neptunium, americium and curium) and fissions them, whereupon the reduction of radioactivity to a level that is actually below that of the original uranium in a little over 300 years:
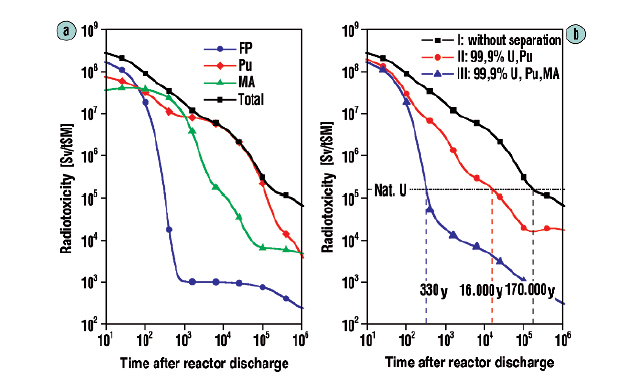
The caption:
(Hartwig Freiesleben, The European Physical Journal Conferences · June 2013)
I note that as is the case of the evacuation of elderly people from nursing homes in the events following the Fukushima event, in the case of the tunnel, the "solution" to the "problem" of the collapsed tunnel, which almost certainly would have not killed anyone from radiation, the fear of radioactivity caused more damage and likely deaths than the radioactivity itself.
Over 4000 diesel trucks, spewing toxic particulates and other carcinogens, hauled tons upon tons of concrete and grout, created in clinker ovens by heating minerals to high temperatures using dangerous fossil fuels, were used to seal the tunnel albeit hardly to the satisfaction of these radiation paranoid morons who think that every radioactive atom on the planet - except for those extracted to make hydrogen perhaps, is a tragedy.
The "828 Underground Nuclear Tests" post was too long, and this one is too.
It's the Fourth of July as I write, in which we celebrate history.
In 2023 history is under attack, but should history continue to exist, let me close with a remark I often make, my personal chant:
"History will not forgive us, nor should it."
Enjoy the holiday.
Hugh_Lebowski
(33,643 posts)Also lets say, offhand/randomly, it's meant to provide 1 exajoule/year.
But you want it to be the only power supply in it's region, so you decide to build in a mechanism to electrolyze H2 from H20 and then burn it for power as needed at night.
Approximately by what degree does the size of the 'farm' have to grow to achieve this?
I already understand that this system seems a stupid undertaking in terms of efficiency, but what do the numbers look like?
Would you have to install 30% more panels to provide 1 exajoule/year and (0 disruptions) for the H2 'battery' or 300% more panels, or 1000% more, etc?
Obviously location matters, so say it's on the equator.
Blues Heron
(5,938 posts)seeing as how night on the equator is about the same length as day is. The efficiency losses would be balanced by the reduced nighttime demand.
NNadir
(33,527 posts)NNadir
(33,527 posts)We not only have the thermodynamic inefficiency of electrolysis itself and the weather - it rains in many places -but the embodied energy of relaxing all the infrastructure for example to prevent the well known phenomenon of hydrogen embrittlement of metals, the energy losses of compression and cooling and liquefying a gas with one of the lowest critical points known.
One cannot do these things with unreliable energy.
I don't have time right now for a BOE calculation, but the solar fantasy is not sustainable already even without hydrogen and even less so with hydrogen. If I have time at some point, I may run a BOE, but I already know it's a worthless but regrettably funded undertaking, pretty much as bad and as useless as all that CO2 sequestration nonsense that flies around, gets funding and does nothing.
The solar industry is pretty good at sucking up money and doing next to nothing.
We spent over a trillion bucks on solar alone in this century for 5 Exajoules per year on a planet with energy demands of over 600 EJ per year and rapidly rising.
It's a junk fantasy.
Hugh_Lebowski
(33,643 posts)So what if we just account for this part: "the energy losses of compression and cooling and liquefying a gas with one of the lowest critical points known".
Also this part: "One cannot do these things with unreliable energy." true but if one has enough H2 reserves, they will have reliable energy. So make an adjustment as needed to assume it will be there, at least within reasonable boundaries.
Roughly how much are we talking about in terms of growing the array to accomplish the stated task of making the supply continuous? 1.5x? 3x? 10x? 100x?
Whenever you have some time ![]()
Blues Heron
(5,938 posts)NNadir
(33,527 posts)The nuclear industry is over 70 years old, and has been attacked by lots of morons who couldn't care less about climate change, air pollution deaths, and the lives saved by nuclear energy.
Prevented Mortality and Greenhouse Gas Emissions from Historical and Projected Nuclear Power (Pushker A. Kharecha* and James E. Hansen Environ. Sci. Technol., 2013, 47 (9), pp 4889–4895)
How about it? After 70 years of nuclear paranoia, how many people were killed by it?
As many as will die today from air pollution? (Where, exactly, does nuclear energy deaths appear in this comprehensive publication?)
Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019 (Lancet Volume 396, Issue 10258, 17–23 October 2020, Pages 1223-1249). This study is a huge undertaking and the list of authors from around the world is rather long. These studies are always open sourced; and I invite people who want to carry on about Fukushima to open it and search the word "radiation." It appears once. Radon, a side product brought to the surface by fracking while we all wait for the grand so called "renewable energy" nirvana that did not come, is not here and won't come, appears however: Household radon, from the decay of natural uranium, which has been cycling through the environment ever since oxygen appeared in the Earth's atmosphere.
Here is what it says about air pollution deaths in the 2019 Global Burden of Disease Survey, if one is too busy to open it oneself because one is too busy carrying on about Fukushima:
Obsessive ignorance is not neutral. It kills people.
NNadir
(33,527 posts)...that stupid journalists use whenever they report on new projects to industrialize wilderness, destroying it, for solar and wind industrial parks.
A "California" as I showed in this post, Cheers and More Cheers! The US is set to add 41 GIGAWATTS of solar power in the next 12 months, applying something called "data" from the California Energy Commission, is 34,886 GWh or 0.125 Exajoules, the latter conveniently but coincidently close to 1/8 of an Exajoule.
Thus we would require 8 "Californias" to produce 1 Exajoule of Electricity, this after half a century of wild cheering for solar energy in California, but the electricity would only be available sporadically because of the effect of a rumored astronomical effect called "night."
I think the "California" is more useful than an equatorial theoretical solar plant, because California has vast stretches of desert ecosystems being converted into industrial parks, as noted in this Guardian article:
How solar farms took over the California desert: ‘An oasis has become a dead sea
A picture of the destroyed desert ecosystem from the article:

I don't know if this picture disgusts the hydrogen morons who fly around here all the time - I expect it doesn't - but it sure as hell disgust me.
For convenience, we'll ignore the cost of shipping water to the ruined solar landscape.
Electrolysis systems are subject to hysteresis, which is a time lag between the time that a electrolyzer first has current applied to it so operational efficiency when the system has been running for a time is further degraded in a temporal sense.
Electrolyzers vary in efficiency in use, but let's use this page from the International Energy Agency, as a yardstick:
IEA, Electrolyzers
This page from 2022 includes the following text:
We'll ignore for a moment that PV solar cells do not produce high temperatures - some years ago I covered a class of neodymium based electrodes for the high temperature electrolysis of steam on another website where I was banned for telling the truth - and settle on 85% thermodynamic efficiency as a working figure, ignoring the related but different concept of Faradaic efficiency.
We are now at 8/.85 = 9.4 Californias.
As it turns out, the US EIA has a nice webpage showing the theoretical minimum energy cost of the compression and liquefaction of hydrogen, as opposed to the real cost but I'm going to this open source paper, Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917, which gives a figure of 10 kWh (36 MJ) per kg of hydrogen, consistent with other references indicating about a 30% energy loss to produce liquid hydrogen having an energy content of roughly 120 MJ/kg.
Since except in places like Greenpeace, where addition and subtraction are considered useless, this means about 70% efficiency since 1.00-0.3 = 0.7.
This suggests that to produce 1 EJ of liquid hydrogen about 9.4/.7 = 13.4 Californias to produce 1 EJ of liquid hydrogen.
Now the hydrogen morons flying around here all want to tell us we also have to pay attention to their useless wilderness destroying wind junk that becomes landfill in about 20 to 25 years, although they love to prattle on about energy intensive (heat requiring) recycling of this junk, which is hardly a wide spread industrial practice for wind and solar junk.
Let's play pretend and say that half of the world energy demand comes from solar hydrogen and half from wind hydrogen.
In 2021 world energy demand was 624 EJ, and is almost certainly higher now. Let's say that world energy demand stabilizes - it won't - at 650 EJ, because in solar and wind speak we can say whatever the fuck we want since soothsaying trumps reality.
This means 325 EJ of solar hydrogen or 2.7 trillion kg of solar hydrogen, 2.7 billion tons. Since 1 EJ of liquid hydrogen is 13.4 Californias, it would take "only" 4,370 Californias (for solar) to produce this very, very, very, very popular scheme.
The number of wind Californias to produce the other 325 EJ is left as an exercise.
I have omitted the embodied energy cost of ripping out all of the world's pipelines and replacing them with pipelines not subject to hydrogen embrittlement.
I think we should get right on it; it should only take a few weeks, no?
We're saved!
I rather enjoyed this calculation and will add it to my journal, like I added the Jimmy Carter adventure in being a nuclear liquidator in the 1950's to my journal, another post you inspired.
Hugh_Lebowski
(33,643 posts)Say you have a solar farm and it's 10,000 acres, and at a normal solar farm efficiency, if the sun shone 24/7, it could produce 1 EJ per year (just made up numbers for sake of discussion).
BUT it has a natural gas plant to supplement for the times when, in reality, the sun doesn't shine i.e. night.
If you wanted to replace the dirty natural gas plant with a component of the solar farm that makes fuel via electrolysis of Hydrogen, to make sure the plant actually produces the 1 EJ it would produce if the sun always shone (H2 as a battery, essentially), how much larger would the farm have to be vs it's original 10,000 acres?
Accounting mainly for 'how much energy do you lose from electrolysis to create an adequate stockpile of H2', and 'how much energy do you lose then burning that H2 at night'.
Blues Heron
(5,938 posts)So you would need a second solar array 167 percent the size of the first to get the second exajoule of H2 you are looking for to keep things going at night.
NNadir
(33,527 posts)A working figure for the amount of dangerous natural gas burned each year is here, 2021 data:
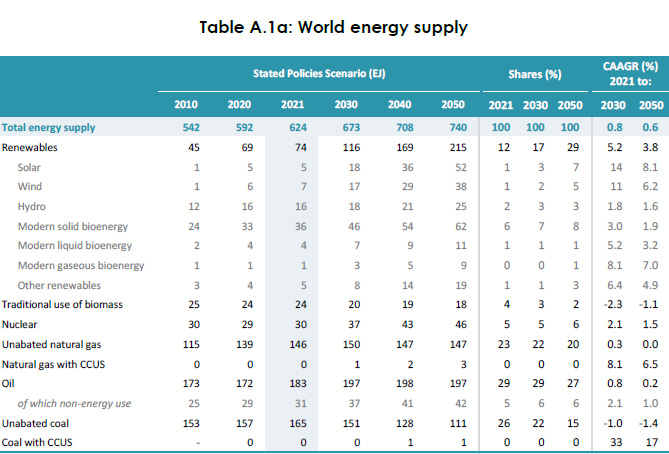
Source: 2022 IEA World Energy Outlook Table A 1a, page 435
I will say this:
According to a cheap google to solar promotion fools, solar cells in the increasingly industrialized destroyed Mohave Desert ecosystem produce, with a tilt mechanism to track, about 6.8 MJ/m^2-day.
This suggests that 1 EJ would require about 40 billion square meters without spaces, or about 40,000 sq km.
This is considerably more than 10,000 acres; closer to 1 million acres.
From the efficiencies for electrolysis in my response, including or not including liquefaction, one can do the rest of this calculation and its left as an exercise. One would of course, need some infrastructure, pipelines, gas (or liquid) storage units, compressors, turbines generators, what have you.
I rather like the exciting new energy unit, the "California," 0.125 EJ, however. It suggests that there are about 5,000 sq km of solar cells already in California, a believable number given the pictures of destroyed wilderness in the Guardian article.
The land area of California is roughly 423,000 sq km.
Hugh_Lebowski
(33,643 posts)My solar farm produces 1 electricity unit.
I take and use that 1 electricity unit to create some H2 as fuel for later like when it's dark.
Later, I burn the hydrogen, to create electricity with.
What % of the 1 electricity unit I started with ... will I then end up with as electrical output from the burning of the hydrogen?
Blues Heron
(5,938 posts)so divide 8 by 13.4 and you get 59 percent
you will have 59 percent of the 1 electrical unit from the burning of the hydrogen. 41 percent losses.
Thats why it takes 13.4 californias to produce an exajoule of H2, whereas it only takes 8 californias to produce an exajoule straight off the panels.
props for the new unit NNadir!
Hugh_Lebowski
(33,643 posts)I.E. the electrolysis/liquifying component.
Not the loss due to 'then burning it to produce electricity' part. That cannot be 100% efficient.
Blues Heron
(5,938 posts)Hugh_Lebowski
(33,643 posts)Blues Heron
(5,938 posts)I think that means usable energy content i.e. what you would get out of a fuel cell. 120 MJ/kg = about 33.3 kWh/kg of H2
Hugh_Lebowski
(33,643 posts)From a chemistry/physics perspective, without accounting for the vagaries of various inefficiencies once you actually 'do something' with that fuel, in this case, convert its stored energy into electricity (one of a number of possible uses, all of which would have some degree of efficiency, and these could vary significantly).
S'why I'm asking ![]()
Blues Heron
(5,938 posts)--snip---
Well-designed fuel cells may operate with 83% to 85% fuel utilization when operated with reformate, and above 90% when operated with pure hydrogen. Note that the current efficiency term in (Equation 3-65) is included in fuel utilization, ηfu in Equation (3-66).
Read full chapter
URL: https://www.sciencedirect.com/science/article/pii/B9780120781423500045
NNadir
(33,527 posts)I think I already more or less answered this question, by showing the thermal efficiency of electrolysis to be roughly 85% at best, although for a real useful measure it matters when the solar energy is produced.
Thus, in a very, very, very (almost uselessly) crude way you would need 1/.85 = 1.18 EJ, not counting compression and liquefaction. If your storage tank was very large and very strong, you may avoid liquefaction, but with liquefaction, 1.18/0.7 = 1.68 EJ. In either case you would still need compression.
Again the embodied energy of the systems, pipes, tanks, compressors are ignored.
This will involve, at 120 MJ/kg LHV about 14 billion kg of hydrogen, and 8 times that mass of water ignoring evaporative losses, because we choose to be sloppy and as crude as possible. Since the molecular weight of hydrogen is 1/8 that of water, you would need, again ignoring evaporative losses from resistance heat, about 112 billion kg of water.
But again, this is uselessly crude, particularly because hysteresis and low current conditions are ignored.
1 EJ represents, as their are 86400 seconds in a day, an average continuous power of 11,600 MW.
Many times, particularly on cloudless days, solar output has a (mildly) Gaussian aspect. Near the most recent Equinox in California, (CAISO) that of this past March, there seems to have been clouds and or rain drifting in around this time, and the autumn equinox was also a little bit noisy, so I selected 9/24/2022, near the autumn equinox for the following graphics showing near the peak, the rough 1/2 height in the morning, in this case 8:00 am and the afternoon half-height, 19:25:



The peak was not quite at noon, but noon is close enough. This is important because the thermodynamic efficiency for the electrolyzers will change with the current.
At noon, you're storing energy, at half height you're breaking even, and you're burning hydrogen before half height in the morning, and after half height in the afternoon. You have about 9 and a half hours where you're storing energy as hydrogen, and 15 and a half hours where you're burning it to keep the power level constant. This will surely increase the 1.68 EJ to a higher number.
This will impact your tank size, and thus your external costs.
How you recover the energy will also have bearing. Presumably you would want to be efficient and use the power plant as a combined cycle device, with a thermal efficiency of around 60%, since fuel cells would need to be very large, get very hot, and would require an enormous amount of fluoropolymers.
At 60% thermal efficiency, achievable with a high end combined cycle plant, this brings you up to 1.68/0.6 = 2.80 EJ (liquefaction).
At the 40,000 sq km/EJ from my previous post, this brings the land area required (Mohave desert) to 40,000*2.8 = 112,000 sq km, about 1/4 the land area of California for 1 EJ produced in a single day at an average continuous power of 11,600 MW.
At the winter solstice a bigger tank would be required, as smaller one at the summer solstice but the "close to the equinox" value should be good for a first approximation, ignoring efficiency changes at high current and low current, embodied energy, etc, etc.
I hope this BOE calculation helps.
Hugh_Lebowski
(33,643 posts)like a battery in order to achieve near 100% reliability through solar/wind. I suspected that to do so would require growing the grid to extreme sizes.
Sounds like you're basically saying ... roughly 3X bigger.
Thank you sir!
NNadir
(33,527 posts)..almost certainly be much worse.
hunter
(38,318 posts)It's the same problem at any scale, from a house in Las Vegas to a regional electric grid.
The average household in Las Vegas uses about 30 kilowatt hours of electricity per day.
If we are being generous, the capacity factor of solar is 33%.
Las Vegas solar panels produce daily, on average, in kilowatt hours, eight times their nameplate wattage. A 4 kilowatt solar array will produce 32 kilowatt hours of power on an average day. Bingo. Enough for a house. (Your mileage may very. Probably for the worse.)
Making a house that gets half its power from solar energy is easy. You get enough solar panels to cover your household at it's maximum demand, say when the air conditioner is running flat out, you are cooking lunch on your inductive stove, you are doing your laundry, and the heat pump water heater is running. That's more than 4 kilowatts, but go with it. Solar panels are inexpensive these days, or so I've heard.
Then you size your batteries to carry you through the evening and into the next morning until the sun is high enough in the sky to start generating electricity again. 26 kilowatt hours ought to do it. Last I looked, $22,000 is about right for those batteries if you have an electrician friend.
When that solar system can't carry you, especially on the colder, cloudier days of winter, you simply rely on fossil fuels to keep you going.
Getting to at least 98% reliable 100% solar power (which isn't that great as it means seven days a year of extreme electricity shortages) is another problem entirely, requiring ludicrous amounts of storage, whatever that storage medium is, be it batteries, hydrogen, magic beans, whatever you've got.
The wretched reality of solar and wind power is that outages occur over large areas as do large surpluses. When you've got baskets full of zucchini your neighbor has baskets full of zucchini too, more than you or they can use. When you don't have zucchini neither does your neighbor.
You've got a similar problem with grain, but grain can be stored for a year without too much fuss. Put it in a silo. It's not so simple with zucchini or electricity. Or hydrogen, for anyone who cares.
Coal and nuclear power don't work that way. When one power plant goes offline other power plants continue on and it's possible to obtain near 99.9% grid reliability. The zucchini is always available, all year.
That's not quite the case with natural gas, which depends upon pipeline supplies. When the pipeline fails so do all the gas plants attached to it., including the gas plants propping up those solar and wind follies. Ask Texas.
Hugh_Lebowski
(33,643 posts)My question was focused around, basically, this part: "Getting to at least 98% reliable 100% solar power (which isn't that great as it means seven days a year of extreme electricity shortages) is another problem entirely, requiring ludicrous amounts of storage ..."
How ludicrous are we talking here?
Also I still struggle to see (even after years of reading NNadir and yourself) how if one takes an area who's energy is 100% sourced from natural gas, and build out a renewable installation that can replace 30% of that natural gas ... how this is not a net-positive effect? You're burning less carbon. Yes it only lasts so long (like anything else), and yes, you need 'land' to do so, but in the net I'm just not quite sure why it's a terrible idea?
These practical concerns need to be factored into the calculations if the alternative is 'replace the gas plant with nuclear': You can get politicians onboard easier, build it out quicker, with much less stringent permitting, and much less NIMBY pushback. It also makes funding simpler I'd imagine, as initial outlay is likely much less.
Even if you run out of money on your renewable installation, you just install fewer panels.
Run out of $$$ on an NPP, and you never end up w/a single KW.
I get that it's a drag that this scenario essentially perpetuates NG usage, but ... didn't you already start that way?
Let's just say I've yet to be entirely convinced that renewables don't make at least reasonable sense in areas with a lot of sunlight or wind, where current needs are mostly or wholly served by fossil fuel plants ![]()
hunter
(38,318 posts)If you have an electric grid where 50% of the energy comes from nuclear power and 50% of the energy comes from fossil fuels and you double the amount of nuclear power, then 100% of your electricity will come from nuclear power.
That's not the case with wind or solar. You hit a very hard wall of diminishing returns in both solar and wind nameplate capacity and any imaginable sort of storage long before you achieve a reliable electric grid.
If you exclude nuclear power or fossil fuels then everything will fall apart long before you reach any sort of stable "renewable energy" economy capable of supporting all 8 billion of us.
Hybrid natural gas / solar / wind energy systems won't save the world.
If we don't quit fossil fuels now billions of us are going to suffer and die as a consequence of global warming.
If we quit fossil fuels for some impossible "renewable energy" utopia then billions more of us are going to suffer and die for lack of food, clean water, and basic shelter.
We, the human race, have painted ourselves into a corner here.
The only energy resource capable of displacing fossil fuels entirely, which is something we must do, is nuclear power.
I didn't come to this conclusion easily. I used to be a part-time radical underground anti-nuclear activist (full-time crazy person) mostly here in California, who burned an unconscionable amount of gasoline in the late 'seventies and early 'eighties on the roads between San Onofre and Humboldt Bay.