Environment & Energy
Related: About this forumThe Manufacture of Solar Cells, Siloxane Discharges, and Methyl Mercury Formation.
Last edited Tue Jul 22, 2025, 10:28 AM - Edit history (1)
The paper I'll briefly discuss in this post is this one: Organosiloxanes in the Photovoltaic Production Industry: Their Sources, Mass Loads, and Actual Contribution to Mercury Methylation in Wastewater Lin Xu, Nannan Liu, Siyao Pei, Fengjiao Jiang, Rusong Zhao, Na Li, and Yaqi Cai Environmental Science & Technology 2025 59 (27), 13983-13991.
It concerns the manufacture, in particular, of CIGS type solar cells, which I contend is not sustainable simply because of the limitations of the world indium and gallium supply.
The paper gives some details of the rather nasty chemistry of solar cell manufacture (of course while declaring the exercise "green" ) which includes, as I was already aware the use of hydrofluoric acid. I consider hydrofluoric acid to be the most scary chemical with which I've worked, and I worked with the war gas phosgene, lots of it.
From the text:
Currently, photovoltaic (PV) energy, as the cleanest energy in the 21st century, is being fast developed, with global cumulative PV capacity reaching 760 GW by 2020 (14) and production volume of photovoltaic materials reaching 134 million tonnes in 2022. (15) In photovoltaic materials, siloxanes have varied applications as cutting fluids, sealants, and hydrophobic coating agents, (16,17) but emission of these compounds during photovoltaics production has not been reported until now. In addition, Hg could exist (∼265 mg/kg) as an impurity of rare-earth metals in photovoltaic materials, especially for the CIGS [Cu (In, Ga) Se2] solar cell. (18) Obviously, the above information indicates the potential coexistence of siloxanes and mercury ions in photovoltaics-production wastewater. As one previous study reported abiotic methylation of mercury, such as by the CH3 group from CH3I, (19) the present study makes the following hypothesis: could siloxanes, with Si atoms bearing abundant CH3 groups, cause methylation of mercury by Si–CH3 in aqueous solution? Some references discussed the possible role of organosilicons in mercury methylation: Nagase et al. found evidence of the production of methylmercury by oligodimethylsiloxanes and polydimethylsiloxanes in laboratory experiments (20 °C, pH = 7), while Frye and Chu concluded that the probability of mercury methylation by methylsiloxanes in aquatic environmental conditions should be extremely low. (20,21) Until now, no apparent correlation between methylmercury and organosilicons has been reported in real environmental compartments. In fact, compared with oxygen, carbon is much more difficult to act as a leaving group in a nucleophilic substitution, and hence, cleavages of the Si–CH3 bond are very rare during transformation of siloxanes. (22) However, as hydrofluoric acid is often used as an etchant for photovoltaic materials, photovoltaic production wastewater contains high levels (up to g/L) of F–, and thus, strong formation of Si–F with exceedingly high bond energy may promote Si–C cleavage. In review of high ecological risks of methylmercury, it was necessary to clarify the potential role of siloxanes in methylmercury formation during photovoltaics production.
As usual, the "green" solar cells are described using units of peak power, watts, a common misrepresentation, in other words a fucking lie, that obscures how much energy solar cells actually produce, given the often reported, but frequently ignored that on average, for half of the day, sunlight is not present because of the existence of something called "night," the length of which varies with latitude, and also because of something called "weather," which is becoming more extreme despite all the cheering for so called "renewable energy."
The capacity utilization of solar cells, depending on location, is on the order, as a worldwide average, of 20% to 25%, rarely larger. This suggests, by calculation using the number of seconds in a year having 365.24 days, at 86400 seconds per day, 31,410,640 seconds per year, that 760 GW of solar cells produces about 6 Exajoules per year - the IEA reports a slightly higher figure, 8 Exajoules - on a planet where humanity consumes close to 640 Exajoules per year and rising.
Guess what? Solar cells are not going to save the world, vast amounts of propaganda to the contrary notwithstanding.
The statement in the text above that I have bolded is not true either. The carbon intensity of solar cell manufacture is not, and never will be, as low as that of nuclear energy. The comparative figures are all over the scientific literature.
The scientists performing the study followed mercury by ICP/MS, siloxanes by GC/MS.
A figure from the text:
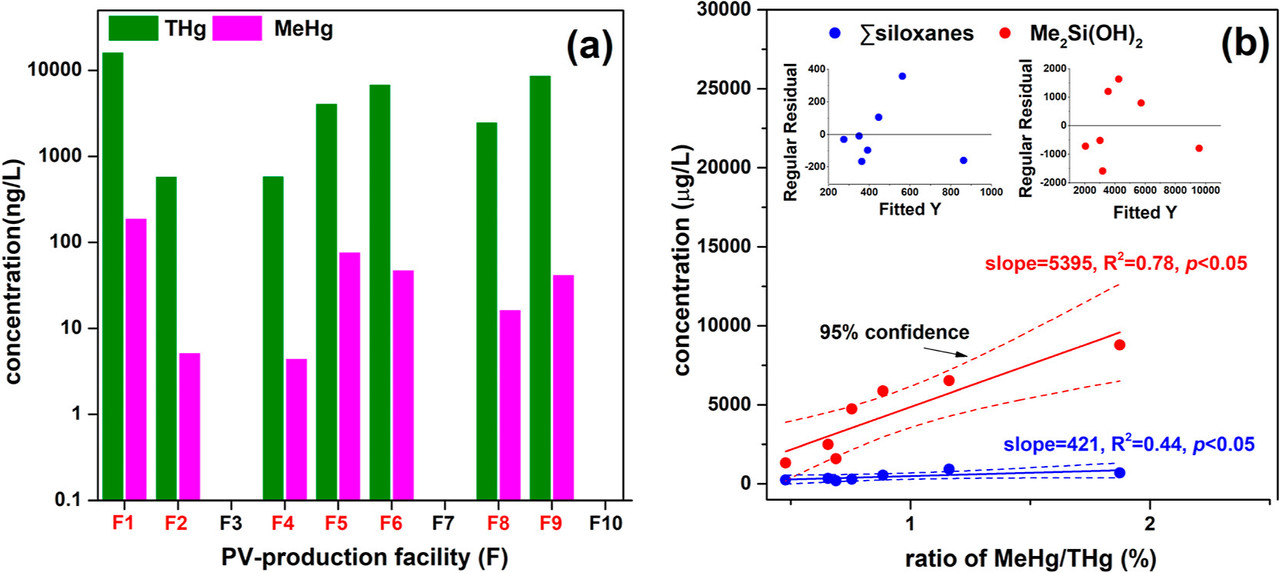
The caption:
The methylation of mercury is, the authors suggest, is abiotic, and is tied to the concentrations of the fluoride ion, which, because of the stability of the silicon fluorine bond, hydrolyzes the methyl groups in the siloxanes. The authors followed the transformation products of the siloxanes by the most sensitive and accurate mass spectrometers in the world, FT/ICR/MS.
The relation between fluoride and mercury methylation is shown in the following figure.
The caption:
So called "renewable energy" is mass intensive, highly dependent on vast mining enterprises. Mercury is not used directly in solar cells, but is an artifact of the mining of the other metals in these cells, in CIGS solar cells, copper, indium, gallium as well the toxic relatively rare nonmetal element selenium, which is an artifact of copper mining, and is recovered from the anode sludge.
I recently reported in this space that the supply of fluorine in China is reportedly limited: Fluorine Flows in China.
So there's that.
We are not going to mine our way of the extreme global heating we are now experiencing.
Have a nice day.