Environment & Energy
Related: About this forumNew materials remove CO2 from smokestacks, tailpipes and even the air
http://portal.acs.org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_ARTICLEMAIN&node_id=223&content_id=CNBP_029012&use_sec=true&sec_url_var=region1&__uuid=b30ba7e3-d19d-4a57-8885-88cf43b87ce0[font size=3]“Carbon Dioxide Capture from the Air Using a Polyamine Based Regenerable Solid Adsorbent”
Journal of the American Chemical Society[/font]
[font size=3]Scientists are reporting discovery of an improved way to remove carbon dioxide — the major greenhouse gas that contributes to global warming — from smokestacks and other sources, including the atmosphere. Their report on the process, which achieves some of the highest carbon dioxide removal capacity ever reported for real-world conditions where the air contains moisture, appears in the Journal of the American Chemical Society.
Alain Goeppert, G. K. Surya Prakash, chemistry Nobel Laureate George A. Olah and colleagues explain that controlling emissions of carbon dioxide (CO2) is one of the biggest challenges facing humanity in the 21st century. They point out that existing methods for removing carbon dioxide from smokestacks and other sources, including the atmosphere, are energy intensive, don't work well and have other drawbacks. In an effort to overcome such obstacles, the group turned to solid materials based on polyethylenimine, a readily available and inexpensive polymeric material.
Their tests showed that these inexpensive materials achieved some of the highest carbon dioxide removal rates ever reported for humid air, under conditions that stymie other related materials. After capturing carbon dioxide, the materials give it up easily so that the CO2 can be used in making other substances, or permanently isolated from the environment. The capture material then can be recycled and reused many times over without losing efficiency. The researchers suggest the materials may be useful on submarines, in smokestacks or out in the open atmosphere, where they could clean up carbon dioxide pollution that comes from small point sources like cars or home heaters, representing about half of the total CO2 emissions related to human activity.
The authors acknowledge the Loker Hydrocarbon Research Institute and the U.S. Department of Energy.[/font][/font]
eppur_se_muova
(36,274 posts)I thought you had to be a subscriber, or login through a subscriber's portal, to see full articles.
caraher
(6,278 posts)I hadn't heard that JACS was among them. I think in some instances authors can pay to have the journal do this.
BeFree
(23,843 posts)That is a priority... clean up coal emissions.
I know, I know, some here don't believe clean coal is possible.
But they can do it. The question is: Can we afford it?
OKIsItJustMe
(19,938 posts)I agree that we need to do something about coal emissions. I also feel we need to do something to actively remove CO2 from the atmosphere (sitting back to let “Nature” take her course will not produce results fast enough.)
However, “cleaner coal” is not what this study seems to be about:
The capture of CO2 from concentrated industrial streams such as exhaust gases of coal burning power plants, cement or aluminum factories, and fermentation plants has gained a lot of attention and has been well described in recent publications.(9-13) While about half of the anthropogenic CO2 emissions are the result of large industrial sources such as power plants and cement factories, the other half originate from small distributed sources such as cars, home heating, and cooking.(14) For those, CO2 capture at the emission source is not practical and/or economical. A possible pathway to deal with these emissions is to capture CO2 directly from the air. One of the advantages of CO2 capture from the atmosphere is that the needed infrastructure can be placed anywhere, preferably where it has the least impact on the environment and human activities or close to CO2 recycling centers.
…[/font][/font]
This adsorbent appears to operate at a relatively low temperature (i.e. 25 °C) while relatively high temperatures (i.e. 85 °C) are used to release the adsorbed CO2.
BeFree
(23,843 posts)Dirty, cheap coal is 50% of why we are having climate changes.
Don't kid yourself. We have to make burning coal cleaner.
Can we afford it? That is the question. The answer is Hell Yes!
So will we? Nah.
OKIsItJustMe
(19,938 posts)…

Source: U.S. Greenhouse Gas Emissions Inventory (y-axis units are teragrams of CO2 equivalent)
…

Source: U.S. Greenhouse Gas Emissions Inventory (y-axis units are teragrams of CO2 equivalent)
…
Still, I agree, we need to do something about coal emissions.
Nihil
(13,508 posts)> After capturing carbon dioxide, the materials give it up easily so that the CO2 can
> be used in making other substances, or permanently isolated from the environment.
I was really excited to read the first part of the post but when I got to the above line,
my smile faded as I realised that this is only concerned with the capture of CO2.
The fuzzy little handwaves above undermine the wonderful progress that has been made
in the capturing technology as there is little demand for CO2 reuse (compared to the
quantity generated every day) and *still* no safe way to make it "permanently isolated
from the environment".
As an academic exercise it is instructive.
As a demonstration of applied chemistry it is very good.
As a solution to "one of the biggest challenges facing humanity in the 21st century"
(specifically the "smokestacks" or "out in the open atmosphere" ) it sucks - literally.
It sucks the CO2 out of the air until saturated then sits down and waits for the
"a miracle happens here" box to complete the work.
kristopher
(29,798 posts)If used to scrub smokestack emissions it is as you say.
However, if the article is accurate it opens some new possibilities that many have heretofore been reluctant to endorse. Concentrated CO2 is a valuable resource but ideas for large scale use have been perceived accurately as unsustainable solutions because the only economically viable source of concentrated CO2 has been seen as coming from previously sequestered sources like combustion of fossil fuels.
If (stress 'if') they've developed an economic low-energy way to concentrate CO2 from the air, then we can start looking at solutions such as algae oil for replacing hydrocarbons; we can also look at methods of sequestration where the point of sequestration is removed from the point of pollution - eliminating or reducing the transportation bottleneck.
Nihil
(13,508 posts)> then we can start looking at solutions such as algae oil for replacing hydrocarbons;
Although this is really just recycling (unless the hydrocarbons are to be used for purposes
other than combustible fuel) but even that is progress over what we have today.
> we can also look at methods of sequestration where the point of sequestration is removed
> from the point of pollution - eliminating or reducing the transportation bottleneck.
Very true.
kristopher
(29,798 posts)It could be a sustainable source of liquid fuel except that any type of volume production requires high infusions of CO2 as 'fertilizer". If we are getting that from a smokestack as is now the practice, then it is really nothing more than a decrease in the carbon intensity of the original fossil fuel. And like you say without enthusiasm, that is progress (but...).
There are, however, sustainable strategies for producing concentrated CO2 - primarily biofuel smokestack emissions such as burning methane from compost. And specific to algae we can also combine it with fish farming and use the algae to scrub their exhalations and re-oxygenate the water.
As can be seen, such precursor processes limit the scale of high-volume production and tie it to the agricultural sector.
OKIsItJustMe
(19,938 posts)[font size=3]28 July 2010
A US company developing a novel way to convert carbon dioxide into plastics is one of six firms receiving a total of $106 million (£68 million) in government funding as the US pushes research converting captured waste carbon dioxide into useful products.
Massachusetts-based Novomer has received $18.4 million from the US Department of Energy (DOE) to develop a process for converting carbon dioxide into polycarbonate polymers that could be used to make plastic bottles.
Since its formation 4 years ago, Novomer has been developing a way of reacting traditional epoxide feedstocks with carbon dioxide from industrial waste streams to form plastics that contain between 40 and 50 per cent carbon dioxide by weight. The company uses a catalyst technology developed by Geoff Coates at Cornell University in New York, US, and employs a cobalt catalyst which chief executive Jim Mahoney says is fairly straightforward to synthesise despite being a relatively complex organometallic compound.
Using the catalyst technology, Novomer can form both high molecular weight (MW) polymers to make thermoplastic polymers that can be used to make plastics, and low MW polymers that can be used to make resins for use as coatings and adhesives. The technology can also be used to convert carbon monoxide into a range of chemicals such as acrylic acid and 1,3 propanediol.
…[/font][/font]
arendt
(5,078 posts)In early 2011, researchers patented a process involving a genetically-modified form of blue-green bacteria that converts sunlight and carbon dioxide directly into diesel fuel.
The process feeds concentrated waste CO2 to a new kind of blue-green bacteria – a highly-engineered type of bacteria, Church said, nothing you would find floating in a pond. The bacteria use the CO2 and sunlight to directly “sweat out” diesel fuel. Church said:
We use very high levels of carbon dioxide that are wastes from industrial products. We use a genetically engineered
^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^
cyanobacteria and an efficient photobioreactor. And these things sum together in something that’s five to fifty times more efficient than any processes built on biomass.
-----------------
One scientist finds a way to scrub the CO2, another scientist finds a way to recycle it into diesel (which can be used to make plastic).
The point is that, the two sides of burning and remaking fossil fuels can now operated in a CLOSED-CYCLE CO2 loop!
We are close enough to doing that that it is a crime to keep subsidizing nuclear.
Saving Hawaii
(441 posts)Saving Hawaii
(441 posts)You also write:
"This adsorbent appears to operate at a relatively low temperature (i.e. 25 °C) while relatively high temperatures (i.e. 85 °C) are used to release the adsorbed CO2."
25C is a good temperature for trees. 85C will hasten oxidization, no? Hmmm....
OKIsItJustMe
(19,938 posts)However, looking back 100,000’s of years, we find that trees (and other natural carbon sinks) can only remove CO2 from the atmosphere at the rate of ~1ppm/1,000 years, and in those days, we didn’t have chainsaws.
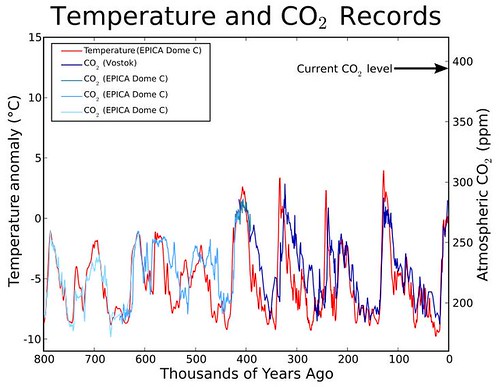
Some scientists believe we need to decrease atmospheric levels of CO2 to 350ppm or lower in a matter of decades.