Science
Related: About this forumThe High Molecular Diversity of Extraterrestrial Organic Matter in the Murchinson Meteorite.
The paper I will discuss in this post goes back a few years, and concerns the reanalysis of the Murchinson Meteorite, one of the most interesting and important meteorites ever analyzed - because of its implications for the origin of life - 40 years after it fell and was discovered in Australia. The paper is this one: High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall (Phillipe Schmitt-Kopplin et al PNAS February 16, 2010. 107 (7) 2763-2768)
The paper is happily open sourced, at least, apparently at the link provided.
The Murchinson Meteorite is of special interest because it contains a large number of amino acids, as do many other extraterrestrial objects, and, of course, as all living things because they are the constituents by which proteins are made. What is different about the amino acids in the Murchinson Meteorite is that many - but not all - of the amino acids are chiral; that is they exhibit the property of being isolated from their mirror images.
Some very basic organic chemistry for those who do not know it:
With one major exception, glycine, and a few minor exceptions, all of the amino acids in living things exhibit this property, which is sometimes called "handedness" since hands are mirror images of one another. This graphic from Wikipedia shows the property nicely:
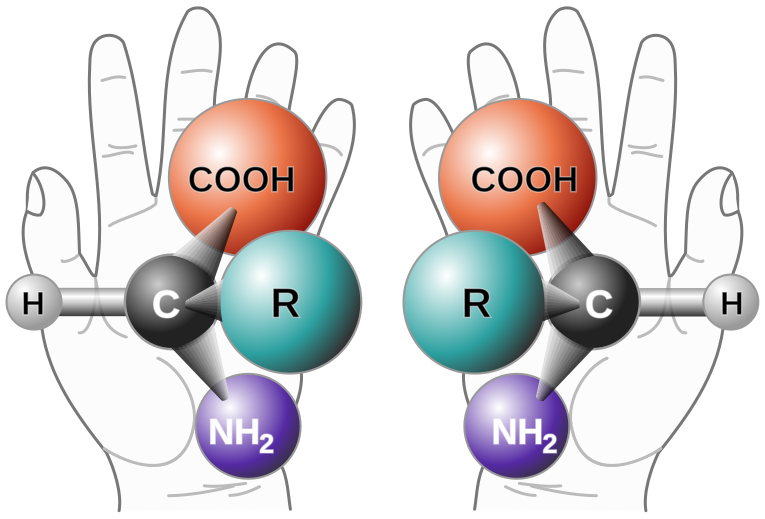
In this picture, three dimensional examples - except if "R" is hydrogen - of the amino acids drawn cannot be superimposed upon one another; they are different molecules, called enantiomers, again, mirror images, featuring the same molecular connectivity but different arrangements in space.
One cannot in general make one enantiomer in the absence of the other in the lab unless one already has some reagent in the reaction medium which is also chiral and chirally pure. (Even with such reagents, the synthesis of a pure enatiomer can be problematic.) A mixture of two enantiomers in exactly a 50:50 mixture is otherwise obtained; we call such a mixture a "racemate." A pure single enantiomer, or a mixture in which is nearly or partially purified with one enantiomer dominating the other is said to be "optically active," since chiral compounds cause plane polarized light to rotate.
Thus chirality needs to have an origin; but one of the great scientific mysteries to still remain is "what is the origin of chirality?" If the origin of chirality is explained, it is presumably easier to explain the origin of life. For many years the origin of chirality was thought to have originated on Earth, but the Murchinson meteorite, where some of the amino acids are present in chiral excess, suggests, surprisingly at the time, that chirality can originate and is known in outer space.
For some time, it was thought that the meteorite was contaminated by terrestrial amino acids when it landed, although there were over 80 different amino acids in the meteorite, whereas living things are generally, again with some exceptions, limited to 20 amino acids, and - another mystery - DNA codes for only 20, with only one known exception, an amino acid known as selenometionine known in certain bacteria.
The fact that the amino acids in the meteorite was in fact extraterrestrial was suggested by the fact that certain "coded" amino acids were missing and again, that there were so many unusual amino acids.
The matter was settled when it was discovered that the isotopic distribution of the constituent atoms in the meteorite, including those in the amino acids was typical of extraterrestrial space and not Earth: Isotopic evidence for extraterrestrial non- racemic amino acids in the Murchison meteorite (Engle and Macko, Nature volume 389, pages 265–268 (18 September 1997))
The authors of the paper cited at the outset here, who reanalyzed parts of the meteorite 40 years after it fell, using more advanced instrumentation than was originally available to the first scientists to analyze it, remark that the focus on amino acids missed something, which was the analysis of things other than amino acids.
From their introduction:
..
...all previous molecular analyses were targeted toward selected classes of compounds with a particular emphasis on amino acids in the context of prebiotic chemistry as potential source of life on earth (10), or on compounds obtained in chemical degradation studies (11) releasing both genuine extractable molecules and reaction products (11–15) often difficult to discern unambiguously.
Alternative nontargeted investigations of complex organic systems are now feasible using advanced analytical methods based on ultrahigh-resolution molecular analysis (16). Electrospray ionization (ESI) Fourier transform ion cyclotron resonance/mass spectrometry (FTICR/MS) in particular, allows the analysis of highly complex mixtures of organic compounds by direct infusion without prior separation, and therefore provides a snapshot of the thousands of molecules that can ionize under selected experimental conditions (17).
Here we show that ultrahigh-resolution FTICR/MS mass spectra complemented with nuclear magnetic resonance spectroscopy (NMR) and ultraperformance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS) analyses of various polar and apolar solvent extracts of Murchison fragments demonstrate a molecular complexity and diversity, with indications on a chronological succession in the modality by which heteroatoms contributed to the assembly of complex molecules. These results suggest that the extraterrestrial chemical diversity is high compared to terrestrial biological and biogeochemical spaces.
Some pictures from the paper:

The caption:
Progressive detailed visualization of the methanolic Murchison extract in the ESI(−
The authors extracted different classes of the compounds by the use of differing solvents:

The caption:
Extraction efficiency of the solvents. (A) Number of total elemental compositions found in ESI(−

The caption:

The caption:
Distribution of mass peaks within the CHO, CHOS, CHNO, and CHNOS series for molecules with 19 carbon atoms. CHO and CHOS series exhibit increasing intensities of mass peaks for aliphatic (hydrogen-rich) compounds, whereas CHNO and CHNOS series exhibit a slightly skewed near Gaussian distribution of mass peaks with large occurrences of mass peaks at average H/C ratio. The apparent odd/even pattern in the and CHNOS series denotes occurrence of even (N2) and odd (N1,3) counts of nitrogen atoms in CHNO(S) molecules in accordance with the nitrogen rule (Fig. S8E).
Since this extremely high resolution FTICR/MS (Fourier Transform Ion Cyclotron Resonance Mass Spectrometer) was utilized in a flow injection fashion, it is not possibly to discern the precise nature of these molecules; it only indicates that there are a wide variety of them. (Some Triple TOF analysis was performed using LC's, but there isn't that much detail in the paper.)
I won't quote any more from the paper; the interested reader can read it on line.
A scientist reader who is interested in the resolution is advised to look in the supplemental info associated with the paper. It also contains some Van Krevelen diagrams, which were originally developed to describe the sources of dangerous fossil fuels, woody matter being responsible for dangerous coal and dangerous natural gas; algae giving rise to dangerous petroleum.
There's a lot of absurd stuff about space aliens that flies around among the increasingly loud fringes. No, space aliens did not build the pyramids and Stonehenge. But it is possible that life is a natural outgrowth of the basic chemistry of carbon, and it is possible that many of the molecules of life, and maybe even life itself, did not originate on Earth.
It may be a matter of quasi-faith, but since we are so hell bent on destroying this planet, it's a comforting thought to think that life might well, and probably does, exist elsewhere in the universe.
Have a nice hump day.
Puzzler
(2,505 posts)Thanks, I’ll forward this to my father, a professor emeritus of (cellular) biology at the University of Victoria.
(FYI: You may Google his name him from my profile, just add “Dr. Michael J.” to my surname)
-Puzzler (sometime amateur astronomer)
NNadir
(33,449 posts)...some of your father's work in other contexts, both in radioprotection and in cell preservation.
He may be aware from general knowledge of the Murchison meteorite.
Puzzler
(2,505 posts)in those fields, especially cryro-biology.
-Pizzler
Vinnie From Indy
(10,820 posts)This is the origin of life on Earth