Science
Related: About this forumHighly Selective Separations of Actinide (and Lanthanide) Elements Using HOPO Ligands.
The paper I'll discuss in this post is this one: Ultra-selective ligand-driven separation of strategic actinides (Rebecca Aberget et al Nature Communications 10, Article number: 2438 (2019)
(If you are interested in this paper, but less interest in my commentary sarcasm and spin, the paper is open sourced and you can read it yourself.)
Some history: In 1944, a fellow named Don Mastick, a 22 year old fellow working on plutonium isolation from the Manhattan project, accidentally swallowed much of the world's supply of isolated plutonium. His story is covered extensively in Eileen Wellsome's book The Plutonium Files: America's Secret Medical Experiments in the Cold War, which is available in many public libraries and places where you can buy books. It's worth reading. Masticks job after swallowing plutonium involved to recover precious plutonium from own his feces and urine, a job he didn't like, and later was able to transfer to a job as a technical accompanist to the bomb parts when they were brought to Tinian to be dropped on Japan. Don Mastick died in 2007; he was 87 years old and for much of his long life was interviewed from time to time about his plutonium eating adventure. Mastick represents the first known case of human ingestion of plutonium.
From the late 1940's through the early 1960's ton scale quantities of plutonium were deliberately vaporized in the planetary atmosphere, probably forming fine aerosol droplets after condensing as a liquid on bomb fragments. Plutonium, among all elements has the third widest temperature range in which it remains liquid, exceeded only to neptunium and gallium. As free unionized oxygen migrated into the blast plasma into the cooling blast core, this plutonium oxidized, probably to PuO2 where it fell, as dust, as a component of "nuclear fallout." If you were born after 1946, you have been living with components of this fall out your entire life, with said concentrations probably peaking around 1963. Concentrations of radioactive elements released by nuclear bomb testing can be pretty much be detected everywhere on Earth, and are often used as tracers for things like water flows, the age of sediments, rates of soil erosion, melt rates of glaciers, etc.
As a result of industrial and war like exposure to actinides like plutonium, and in an effort to make radiotherapeutic drugs for cancer patients - still very much an active area of research - scientists became interested in physiological chelation of plutonium in biological systems, particularly with a goal of removing from animals and humans, in cases of accidental or experimental (or war time) exposure.
As I was researching the origins of the HOPO ligand mentioned in the title of this post, I came across a review article in my files where it is discussed in some detail, this one:
Rational Design of Sequestering Agents for Plutonium and Other Actinides. (Patricia Durbin et al Chem. Rev. 2003 103 11 4207-4282) Here's a graphic from that paper in which the scheme for the synthesis of HOPO is presented, using spermine as starting material:

Spermine, named because it was first isolated from human semen, is a constituent of all eucaryotic cells, where, among other things, it serves to stabilize the geometry of DNA. Metabolically it derives from the decarboxyation of ornithine, itself derived from the genetically coded amino acid arginine by deguanidation.
Anyway. Patricia Durbin, one of the authors of this review, passed away in 2009 at the age of 81. She was an important scientist working at UC Berkeley/Lawrence National Laboratory on the biological chemistry of the actinides, and was considered one of the most prominent health physicists of her time.
Her scientific obituary is here: Dr. Patricia Durbin
Here is an early paper in which she published about the discovery of the HOPO ligand:
Specific Sequestering Agents for the Actinides. 28. Synthesis and Initial Evaluation of Multidentate 4-Carbamoyl-3-hydroxy-1-methyl-2(1H)-pyridinone Ligands for in Vivo Plutonium(IV) Chelation (Durbin et al J. Med. Chem.1995 38 14 2606-2614)
Publication in the Journal of Medicinal Chemistry shows that this ligand was originally designed for medical treatment of people who, like Don Mastick, are unintentionally (or deliberately) exposed to plutonium in order to remove it. It appears however, that the utility of this compound extends to possible industrial use, hence the paper cited at the outset of this post, the Nature Communications paper.
From the introduction to the paper:
After discussing the difficulties of the current approach to actinide separations, which are currently only industrial in liquid/liquid extraction settings, and detailing the difficulties of designing extractants for these purposes, the authors continue:
Actinium-225 is considered to be a very important tool in cancer therapies, but it's availability, which involves bombardment of thorium with high energy protons at Oak Ridge National Laboratory, has been limited, in part by the difficulty in separating it from a wealth of side products in what is essentially a proton driven fission event.
Actinium-225 has a half-life of about 10 days, and rapidly decays, through a number of daughter atoms to bismuth-209, the metastable natural isotope of this monoisotopic element. Attached to a ligand designed to bind to cancer cells, it effectively kills them.
Here's a graphic from the paper demonstrating some of the chemistry of this remarkable ligand:
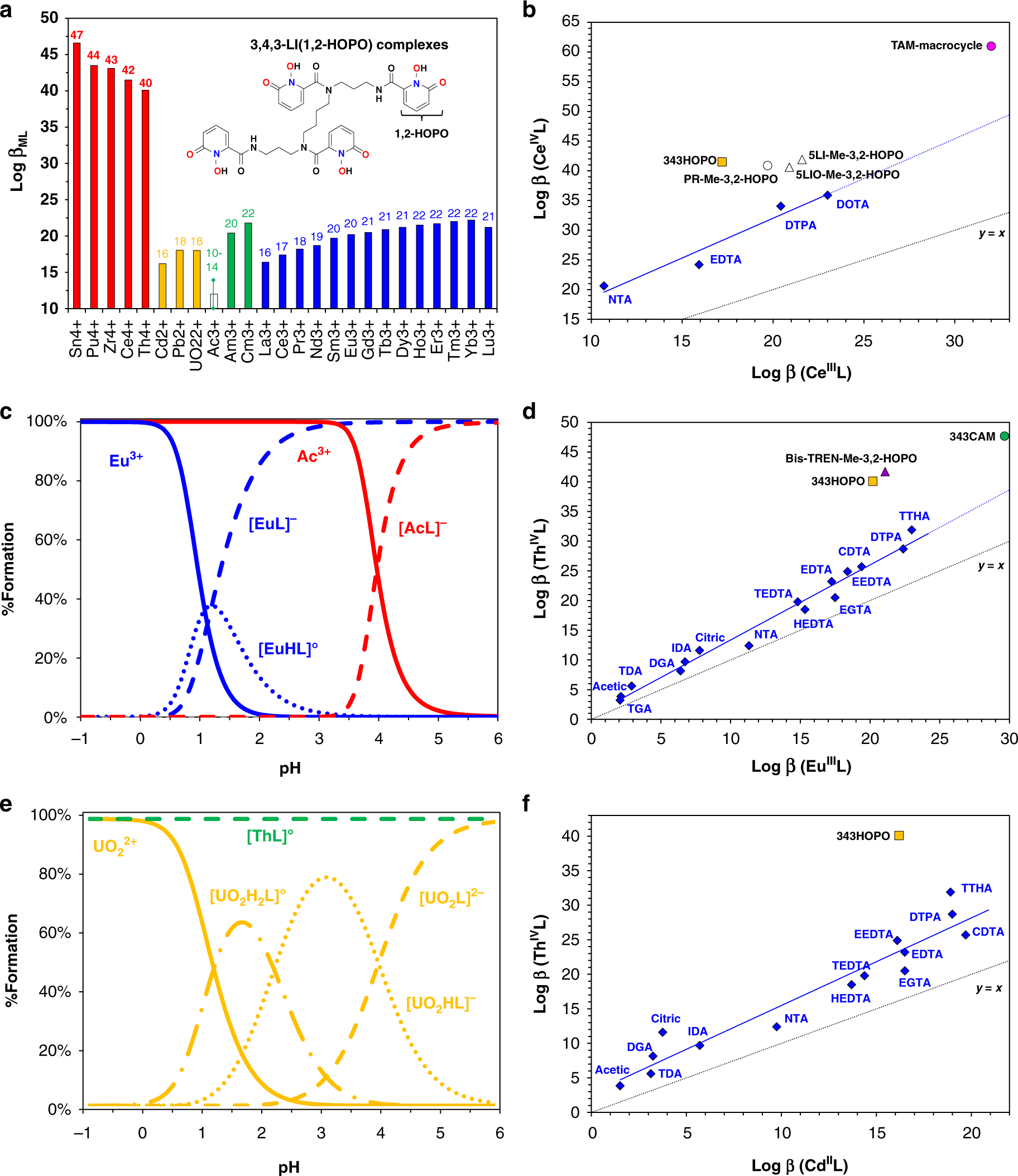
The caption:
I will not produce more texts or graphics from this most interesting paper, since, again, if interested, one can easily access the full paper since it is open sourced at the link above.
Let me say this: In my long considered opinion, the only viable path to addressing climate change passes through the chemistry of the actinides, and indeed the only viable path to getting access to nuclear weapons out of the hands of crazy people by eliminating all or most access to everyone, sane or otherwise - particularly as we can see that a psychopath with a weak education, poor moral development, with puerile emotions and marginal intelligence has gained access and control over the US Nuclear Arsenal - is to make more plutonium, not less of it.
I advanced an argument of why this is true elsewhere: On Plutonium, Nuclear War, and Nuclear Peace, and, although I have expanded my views on what I wrote six years ago, this covers the basic reasoning. It is absolutely critical, pun intended, that we increase world inventories of plutonium-238 and plutonium-240 to minimize the potential for nuclear war.
In doing this, we can also eliminate, using the uranium already mined for centuries, the use of dangerous petroleum, dangerous coal and dangerous natural gas.
The chemistry of the HOPO ligand, after the fashion of medicinal chemistry's SAR approach can be modified in a number of ways to make things like liquid membranes to further refine these separations. To me this interesting expansion on the utility of this ligand from its original purpose of in vivo decontamination to potential industrial utility is worthy of deep consideration.
This work explores the possibility of using simple and clean redox chemistry, including but not limited to, electrochemistry, to provide what may be a continuous process (as opposed to batch processes) for the recovery of valuable elements from used nuclear fuels.
This is important and creative work.
I trust you're having a pleasant weekend.