Carbocations Generated Electrochemically from Carboxylic Acids.
The paper I'll discuss in this post is this one: Hindered dialkyl ether synthesis with electrogenerated carbocations (Baran et al, Nature 573, 398–402 (2019)).
Last year my kid was in France where he was working with some organosilanes and he asked me to scan and email an organic chemistry textbook and some problem solutions so he could teach himself some organic chemistry. He told me he was thinking of taking some organic chemistry courses for one of the minors he was considering.
I said, "Well that's OK with me, but if you take courses in organic chemistry, just don't fall in love with it."
Medicinal chemistry is a dying field in the United States, and with it, the economic value of degrees in chemistry focusing on organic chemistry. That's really terrible, since organic chemistry is such a beautiful science and the history of organic chemistry in the United States is sublime.
It's been some decades since I've worked in the field, but I recall how very much alive I felt in those days, young, newly married to the woman of my dreams and still going to work on the weekends not because I had to do so, not because anyone was paying me to do so, but simply because I loved doing it.
I suppose on a certain level a knowledge of organic chemistry still permeates my day to day life and work, but it's peripheral; I'm sure I've forgotten more than I know.
But today I came across this beautiful and in someways earth shattering beautiful little paper in a major cross disciplinary scientific journal where one doesn't see that much organic synthesis anymore, Nature. (There is a Nature Journal called Nature Chemistry, but I don't read it all that much unless some reference drags me there.)
From the introduction:
Hindered ethers are of high value for various applications; however, they remain an underexplored area of chemical space because they are difficult to synthesize via conventional reactions1,2. Such motifs are highly coveted in medicinal chemistry, because extensive substitution about the ether bond prevents unwanted metabolic processes that can lead to rapid degradation in vivo. Here we report a simple route towards the synthesis of hindered ethers, in which electrochemical oxidation is used to liberate high-energy carbocations from simple carboxylic acids...
I haven't kept up on things, but right off the bat that strikes me as a big deal, carbocations from simple carboxylic acids.
...These reactive carbocation intermediates, which are generated with low electrochemical potentials, capture an alcohol donor under non-acidic conditions; this enables the formation of a range of ethers (more than 80 have been prepared here) that would otherwise be difficult to access. The carbocations can also be intercepted by simple nucleophiles, leading to the formation of hindered alcohols and even alkyl fluorides. This method was evaluated for its ability to circumvent the synthetic bottlenecks encountered in the preparation of 12 chemical scaffolds, leading to higher yields of the required products, in addition to substantial reductions in the number of steps and the amount of labour required to prepare them.
Here's the stuff from the world in which I used to live:
The Williamson ether synthesis3,4 is a long-established method by which to synthesize primary alkyl ethers via SN2 substitution (Fig. 1a). However, in contexts involving secondary or tertiary alkyl halides the reaction often derails, leading to elimination byproducts or to no reaction at all. Hindered ether 1, which is a key intermediate in the synthesis of an aurora kinase modulator, exemplifies this commonly faced challenge. Despite the documented utility of hindered ethers1,2, very little progress has been made in facilitating access to them. The alternative workhorse method, the Mitsunobu reaction, also fails in such settings owing to the steric demands of the SN2 process and the pKa requirements of the nucleophile5...
The Mitsunobu reaction is always a favorite, since it involves a reagent known as DEAD, (diethyl azodicarboxylate).
The pictures tell the story:
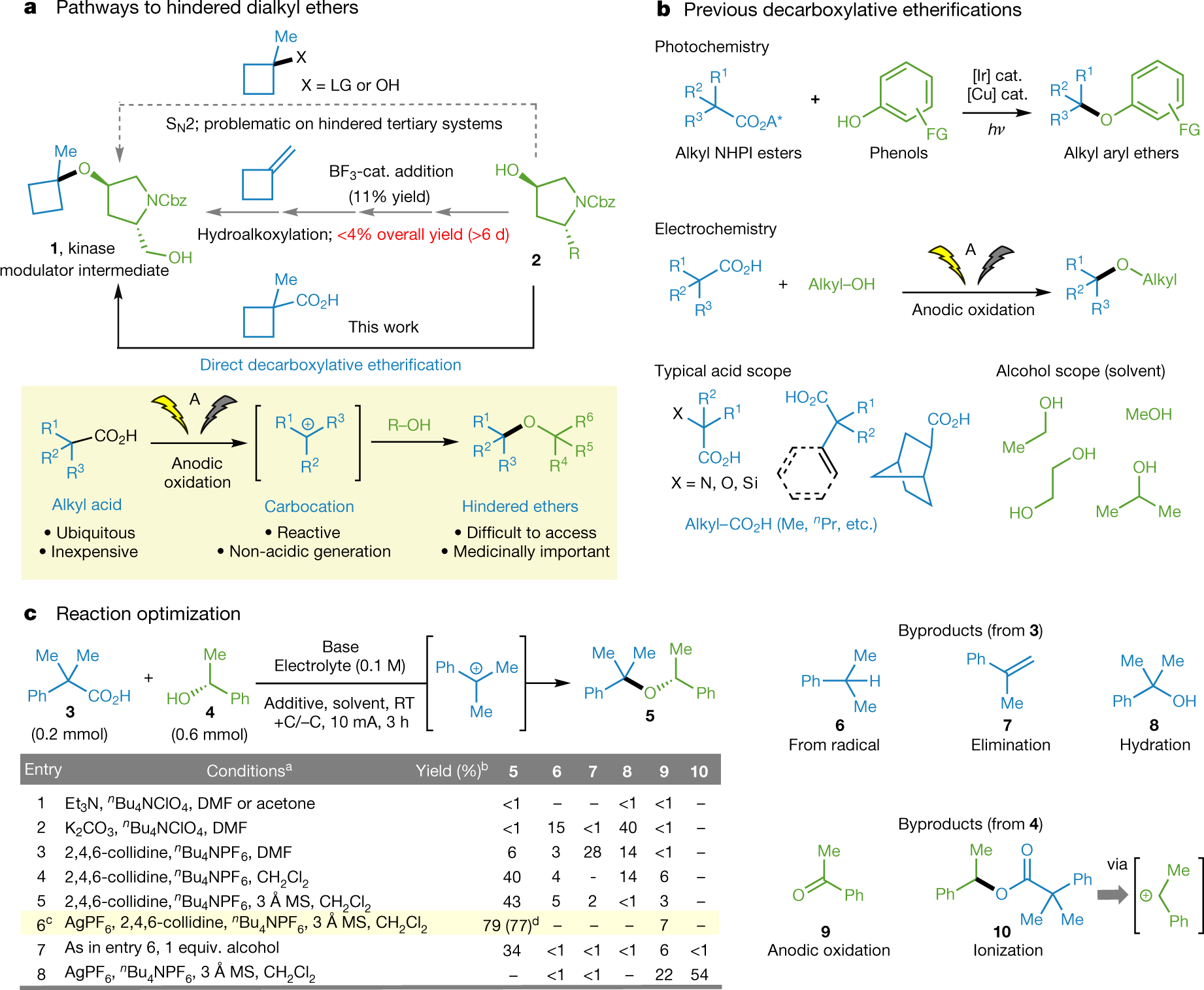
The caption:
a, The synthesis of hindered ethers is a long-standing challenge in organic synthesis. A; constant-current electrolysis; cat., catalyst; Cbz, carboxybenzyl; LG, leaving group. b, Historical context and previous strategies for decarboxylative etherification. FG, functional group; NHPI, N-hydroxyphthalimide. c, Development and optimization of hindered ether synthesis depicted through electromechanistic analysis. aCompound 3 (0.2 mmol), 3.0 equiv. of alcohol 4 (except where designated). bYield based on gas chromatography. All entries were performed in triplicate. cConditions: acid 3 (0.2 mmol), alcohol 4 (0.6 mmol), AgPF6 (0.3 mmol), 2,4,6-collidine (0.6 mmol), nBu4NPF6 (0.1 M), 3 Å molecular sieves (150 mg), dichloromethane (CH2Cl2; 3 ml), current (I) = 10 mA, 3 h. dIsolated yield. DMF, N,N-dimethylformamide; RT, room temperature; +C/−C represents the graphite electrodes.
Actually, this chemistry is quite old according to the authors:
This class (Fig. 1a, yellow inset) stems from the oldest synthetic organic electrochemical reaction, the Kolbe dimerization, which was discovered9 in 1847. In the so-called interrupted Kolbe variant, known as the Hofer–Moest reaction10, electrolytic oxidation of a carboxylic acid under mildly alkaline conditions generates a carbocation that can be captured by incipient nucleophiles10,11,12,13,14,15,16,17,18. A distinct advantage of this reaction is the non-acidic generation of high-energy carbocations directly from carboxylic acids.
It's chemistry with which I'm unfamiliar, for some reason I've always looked askance at electrochemistry, at least until recently, when I have come to regret my intellectual laziness in this regard.
Some structures they synthesized:

The caption:
See Supplementary Information for literature routes. bAgSbF6 (0.3 mmol) instead of AgPF6. cDBU (1,8-diazabicyclo[5.4.0]undec-7-ene; 0.6 mmol) instead of 2,4,6-collidine. dKSbF6 (0.3 mmol) instead of AgPF6. eAlcohol as limiting reagent, conditions: alcohol (0.15 mmol), carboxylic acid (0.45 mmol), AgClO4 (0.6 mmol), 2,4,6-collidine (0.675 mmol), nBu4NClO4 (0.2 M), 3 Å molecular sieves (100 mg), CH2Cl2 (2 ml), I = 10 mA, 3 h. fAgClO4 (0.6 mmol) instead of AgPF6, nBu4NClO4 (0.1 M) instead of nBu4NPF6. g4.0 or 6.0 equiv. alcohol. h1.5 ml CH2Cl2, I = 7.5 mA, 4 h. i nBu4NClO4 (0.1 M) instead of nBu4NPF6, no AgPF6. jReaction performed in triplicate; yield is average of three runs. kSee Supplementary Information for more examples. Boc, tert-butyloxycarbonyl; d.r., diastereomeric ratio; MS, molecular sieves; Ts, tosyl.
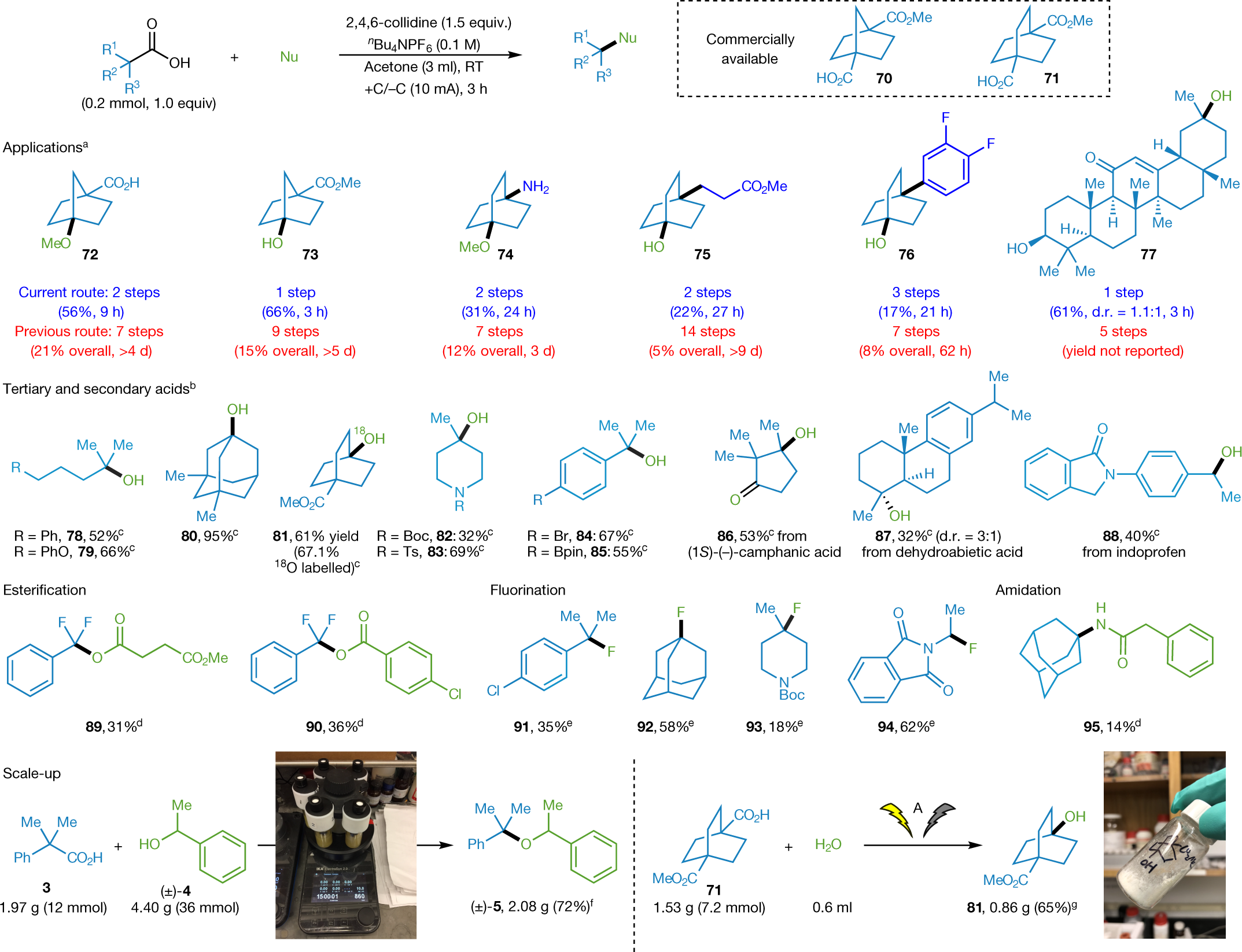
The caption:
See Supplementary Information for literature routes. bSee Supplementary Information for more examples. cH2O (0.1 ml) as nucleophile. dCarboxylic acid or phenylacetonitrile as nucleophile (0.6 mmol, 3 equiv.), conditions: AgClO4 (0.6 mmol, 3 equiv.), 2,4,6-collidine (0.6 mmol, 3 equiv.), nBu4NClO4 (0.1 M), 3 Å molecular sieves (150 mg), CH2Cl2 (3 ml). eKF (0.72 mmol, 3.6 equiv.) as nucleophile, conditions: 18-crown-6 (0.72 mmol, 3.6 eq), AgClO4 (0.6 mmol, 3 equiv.), 2,4,6-collidine (0.6 mmol, 3 equiv.), nBu4NPF6 (0.1 M), 3 Å MS (150 mg), CH2Cl2 (3 ml). fConditions for scale-up to (±)-5 (each reaction): 3 (2.4 mmol), (±)-4 (7.2 mmol), 2,4,6-collidine (3.6 mmol), nBu4NClO4 (0.1 M), 3 Å molecular sieves (450 mg), CH2Cl2 (9 ml) +C/−C (10 mA), RT, 15 h. gConditions for scale-up to 81 (each reaction): 71 (1.2 mmol), H2O (0.1 ml), 2,4,6-collidine (1.8 mmol), nBu4NPF6 (0.02 M), acetone (9 ml), +C/−C (10 mA), RT, 12 h. Nu, nucleophile; pin, pinacolato.
The conclusion of the paper strikes me as something of an understatement:
It is anticipated that the mild electrogeneration of carbocations reported herein will find use in numerous settings in which standard SN2 and carbocation-based approaches are unsuccessful in forming hindered functionalized carbogenic frameworks.
This is by the way, molten salt chemistry, in the extreme, since a key reagent is nBu4NPF6. Electrochemistry in molten salts would be a very big deal in a sane world, not the one we live in, a world where organic chemistry would regain some value.
I know, I know, I know...it's very esoteric...very esoteric...
It's very esoteric, but in a time of so much tragedy, children in cages, a destructive nutcase running the country, it is well worth finding some peace in the knowledge that there
still are wonderful and beautiful things left, even in this country.
I hope you will enjoy a very pleasant up coming weekend.


