Science
Related: About this forumElectrochemical Molecular Switches for the Capture and Release of Uranium.
The paper I'll discuss in this post is this one: Redox-switchable carboranes for uranium capture and release (Gabriel Ménard et al, Nature volume 577, pages 652–655(2020))
According to a DOE report, DOE/EM-0275 as of 1996, the US government had in stock about 585 million kg of depleted uranium, beyond the 25 million kg enriched to about 3% in U-235, about 610 million kg.
A kg of plutonium, the starting material for which is depleted uranium, contains about 80.3 trillion joules of energy, completely fissioned.
The world was, as of 2018, consuming about 600 exajoules (600 million trillion joules) of energy each year.
Recently there have been efforts by a number of companies, one of the most well known being Bill Gates' Terrapower, to commercialize "breed and burn" type reactors that transmute depleted uranium into plutonium in situ.
It follows that this US inventory is sufficient, at current levels of energy demand to power all the world's energy for about 80 years, no dangerous natural gas, no dangerous petroleum, no coal mining for energy purposes. Of course, there are other uranium inventories elsewhere in the world. In addition, a side product of the useless wind industry and the electric car industry, both of which depend on access to iron neodymium boride magnets, often doped with dysprosium - lanthanides - is the radioactive element thorium. Collected from lanthanide mile tailings, dumps, in which the thorium has been partially refined, this thorium is also a valuable nuclear fuel. It is reasonable to say that in a "breed and burn" powered world it would be unnecessary to mine any fuels for several centuries.
Fracked rock, which has been eternally pulverized for a few decades of "good times" by all of us self declared "green" people also represents a potential source of uranium: The radon dumped by the gas industry in Pennsylvania's Reading Pronge gas fields indicates that this pulverized rock, over which water may flow for millenia upon millenia, is also a potential uranium source.
Finally, since that establishment of oxygen in the planetary atmosphere, a continuous uranium cycle has been established in the planetary oceans; they contain about 4.5 - 5 billion tons of uranium.
There has much discussion of refining uranium from dilute sources, seawater, run-off from uranium mine tailings, and natural uranium formations both for the purposes of obtaining fuel as well as to remediate areas of anthropomorphic contamination or natural uranium flows. Uranium is a chemotoxic element, notably having effects on renal and other tissues. Many thousands of papers on this subject have been published in the scientific literature; I almost certainly have hundreds in my personal electronic files. Many of these papers concern organic resins, notably amidoxime functionalized resins. There are also inorganic species that have been advanced for this purpose. What is of interest about this laboratory scale material is that it can more or less breathe uranium, in effect "inhale" and "exhale" it by the application of electrical currents.
From the introduction:
Closo and nido refer to something known as the "Wade-Mingo" rules, and refer to the presence of a complete platonic solid structure, in this case icosahedral symmetry, having all vertices represented, closo or one vertex missing, nido. (The symmetry of in these cases is not truly icosahedral, since the symmetry is "disturbed" or "degraded" by the presence of the functionalized carbon. The carbon in this boron hydride structure is functionalized with diphenylphosphine oxide.
Figure 1:
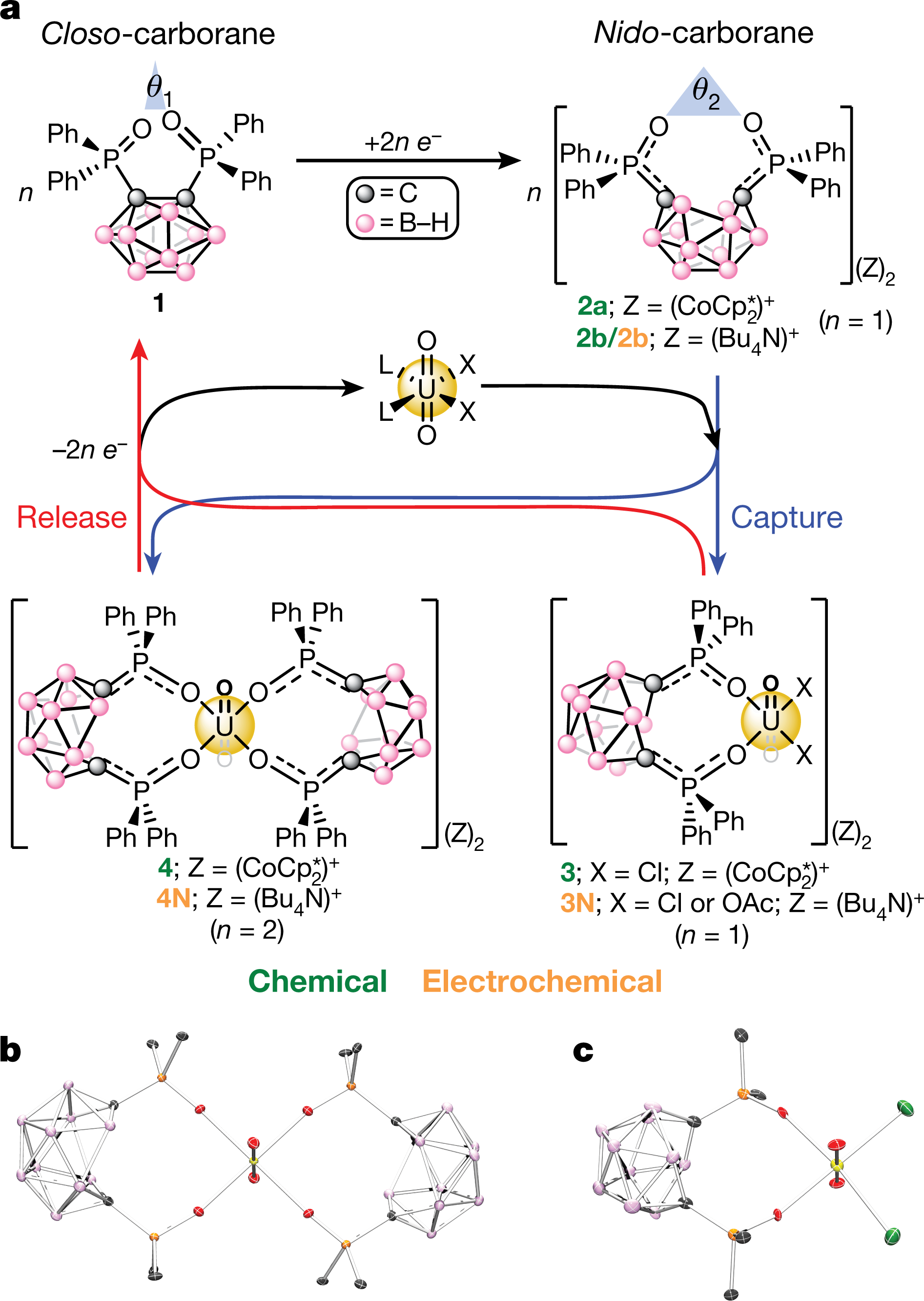
The caption:
a, General chemical or electrochemical mono- or bi-phasic capture of uranyl from UO2X2L2 (X = Cl−, OAc−; L = THF, Ph3PO) using the reduced ‘open’-cage nido-carboranes (2a/2b) generated by reduction (for example, CoCp∗2CoCp2∗ or negative bias) of the ‘closed’-cage closo-carborane (1). The corresponding relative bite angles (θ
Many of the experiments described in the full paper take place in organic solvents, which of course, is not seawater, but nevertheless the system is definitely quite interesting, and one can imagine modifications.
Anyway, the system operates electrochemically.
Figure 2:
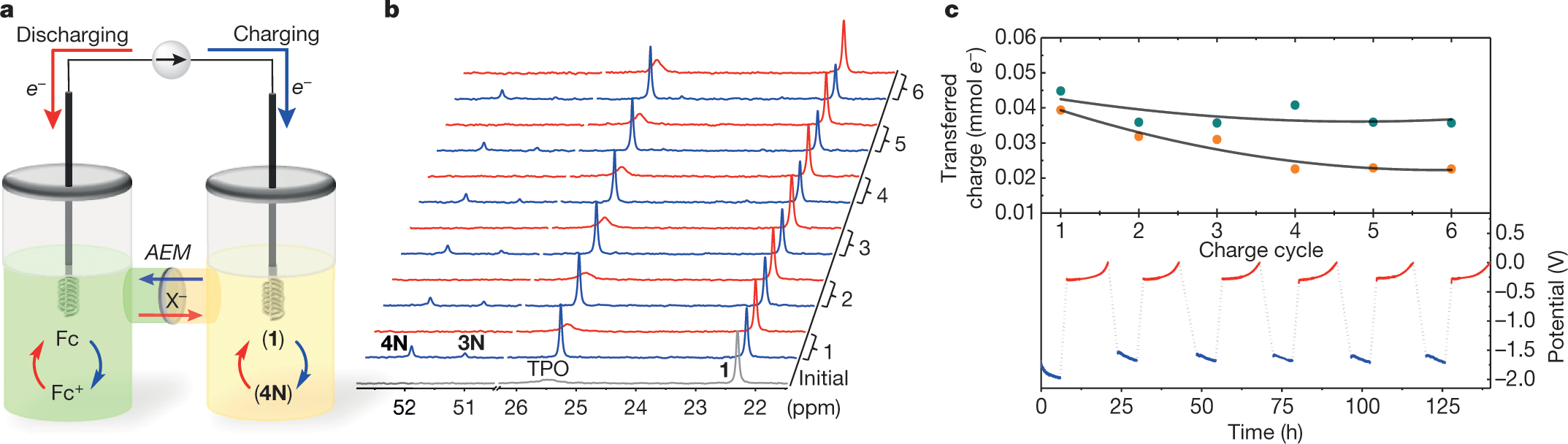
The caption:
The issue of organic solvents is addressed as shown in figure 3, which essentially is an extraction procedure.
Figure 3:
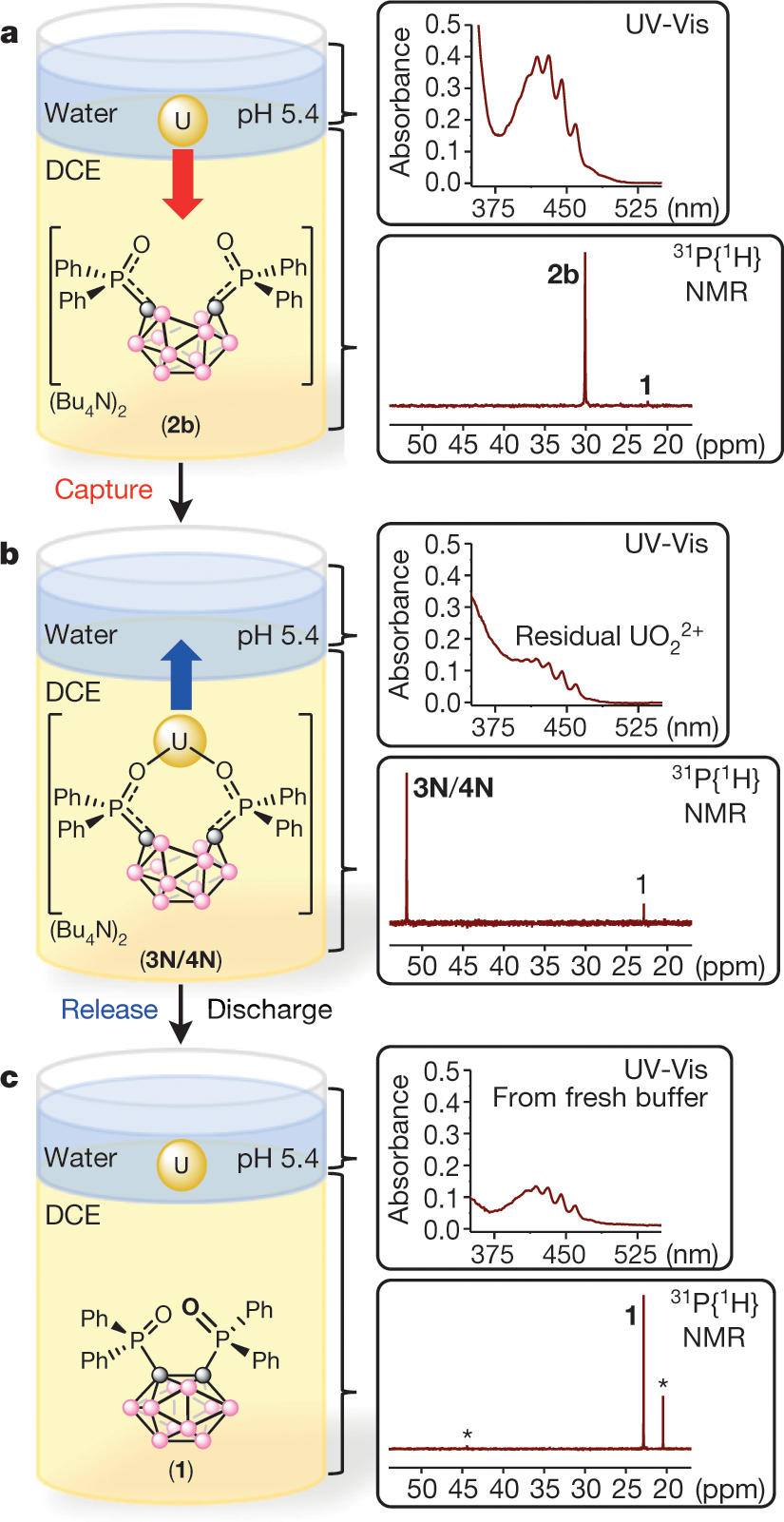
The caption:
Note that exposure to organic solvents would not be acceptable unless the organics were destroyed by subsequent processing. One such available approach to processing would involve subjecting the resultant aqueous solution to supercritical conditions, whereupon the solvent residues would be oxidized to carbon dioxide and the water reduced to hydrogen.
This is a lab scale procedure, and it seems to me that a number of issues need to be addressed before anything like this could be run on an industrial scale. Then again, as stated at the outset, the "breed and burn" concept means that there is really no need to obtain more uranium than has already been mined, at least for several centuries, so there's plenty of time to do that, to make nuclear energy essentially inexhaustible. (At the end of my life, it does seem that ultimately fusion energy may be viable, but current isolated uranium might make the world survivable in the interim.
It's a nice little interesting paper, I think.
Have a nice day tomorrow.
eppur_se_muova
(41,164 posts)That might diminish the VOS problem considerably. I assume they've already considered that, since they mention chelating polymers in the intro.
Bookmarking the paper for later reference, just in case I ever manage to find another job in chemistry.
NNadir
(37,419 posts)These are known, of course, but not really commercial.
I'm not sure that I would consider linking this type of system to a polymer. It is of course - one sees more and more of this particularly in molecular biology based therapeutics, ADC's and the like - possible to imagine a linker, perhaps bound to one of the phenyl groups of the phosphine, and if the linker is conjugated, well maybe. This may or may not address the issue of insolubility in water, I can't say.
Another option however is utilizing filtration type systems by attaching the system to a porous material.
Two summers ago, in his first internship in France, my son worked in an area about which I'd never heard, polymer derived ceramics. These can be utilized to produce porous structures rather like the glass frits that are found in organic laboratories, in things like Buchners, Schlenk tubes and the like.
Also, there is increasingly a lot of work being done on a type of ceramic popularized and developed by Michel Barsoum at Drexel, the so called MAX phases, which are machinable, exhibit some flexibility, are highly ordered, and electrically conducting.
This is a wild speculation on my part, but the advantage of polymer derived ceramic systems as I understand them is that they can be constructed so as to exhibit porosity. MAXenes are two dimensional monolayer carbides or nitrides that exhibit conductivity.
Perhaps in nanostructuring systems, exploiting all these technologies in a synergistic way, we could go somewhere with this interesting technology.
We have plenty of time to do it, because as noted in the OP, the amount of uranium already refined represents an enormous reserve of energy, as does the thorium partially refined by the removal of the lanthanides in the native ores.
I doubt that on this planet we are smart enough to get it, because we're all wrapped up in emotional mysticism, fondness for ignorance, and driven by fear, but we need not obtain any more uranium in order to save the world. We certainly have enough plutonium to set up the spark plug for the system.
It would simply be a matter of us coming to our senses, something that is, admittedly, a long shot. My feeling is that we'll just keep building wind turbines until we reach 500 ppm, still claiming, in exercises of pure idiocy, that nuclear power is "too dangerous" and acting as if climate change and air pollution are not "too dangerous."
cstanleytech
(28,277 posts)automobiles? Given your prior postings regarding the current pitfalls of building high capacity batteries we will still be up the creek right?
NNadir
(37,419 posts)...actually have the effect of raising thermodynamic efficiency over what we routinely accept (30-50%) by utilizing, rather than rejecting waste energy. Thermodynamic efficiency depends on path. It is generally thermodynamically wasteful, at least as currently practiced, to utilize nuclear energy simply to produce electricity.
Electricity is a thermodynamically degraded form of energy, inherently, and worse when utilized to charge batteries.
I don't have a lot of time right this moment, and may wish to invest what time I do have on other topics, but I will try to get back to you with more details. If I don't, and you're interested, feel free to remind me some weekend.
I should note however, my position, long stated, that there is no form of the car CULTure that is sustainable, not nuclear powered or otherwise.
cstanleytech
(28,277 posts)StevieM
(10,578 posts)Do you see any potential for them at all?
NNadir
(37,419 posts)I was/am not particularly familiar with this technology, and as such, I picked up a few review articles on the topic and quickly scanned them.
Mostly I focused on this one, from a Canadian Institution, not because of its title, but because it has a nice easy to read quick overview of the technology,
A review of all‐vanadium redox flow battery durability: Degradation mechanisms and mitigation strategies (Xiao‐Zi Yuan, Chaojie Song, Alison Platt, Nana Zhao, Haijiang Wang, Hui Li, Khalid Fatih Darren Jang, Int J Energy Res. 2019;43:6599–6638.
...and this one...
A high power density and long cycle life vanadium redox flow battery (H.R. Jiang, J. Sun, L. Wei, M.C. Wu, W. Shyy, T.S. Zhao Energy Storage Materials 24 (2020) 529–540 Volume 24, January 2020, Pages 529-540)
Although I appreciate you drawing my attention to them, the topic does nothing to mitigate my general feeling about batteries in general, which are generally supposed to offer hope that the grotesque failure of so called "renewable energy" to address climate change can be mitigated by band-aid after band-aid after band-aid applied with huge dollops of wishful thinking.
With respect to so called renewable energy, a battery does two things: (1) It raises the already unacceptably low energy to mass ratio of this pernicious technology on which we have bet the planetary atmosphere. (2) It rejects heat energy (entropy) to the atmosphere, thus wasting energy.
One doesn't need to even read the full text of the second paper cited, to get to the second point. It's in the abstract:
These energy losses to sustain an already trivial form of energy are treated as a victory. They are not, because electricity is not primary energy, but is rather a thermodynamically degraded form of energy by its very nature. The batteries simply add another layer (two layers actually) to the thermodynamic losses associated with electricity.
(I am currently working on writing an article for this space on the electrochemical reduction of carbon dioxide to give multicarbon molecules during which I may discuss an alternate approach designed to store energy by capture of the energy losses to thermodyanmics inherent in all heat engines, i.e. raising their efficiency. I may get it up today, depending on the flow of time and responsibilities.)
Now, for sure, there are local applicability to batteries. While I object vociferously to exajoule scale battery storage, a battery or fuel cell device to provide immediate back up for systems that require continuous operation, cell phone towers, lighting in large buildings during power outages, etc. I am far less sanguine about devices such as electric cars, which to my thinking, represent an environmentally unacceptable abomination.
Thanks for your question though. I very much appreciate questions that make me look into something about which I know nothing or very little.
hunter
(40,416 posts)We ought to be rebuilding our cities such that most adults are not forced to own a car.
The "freedom" of automobile ownership is an illusion.