Science
Related: About this forumMore Lipstick on the Gas Pig: Dry Reforming Methane with "Solar" Thermal Energy.
The paper I'll discuss in this post is this one: Photothermocatalytic Dry Reforming of Methane for Efficient CO2 Reduction and Solar Energy Storage Shaowen Wu, Yuanzhi Li, Qianqian Hu, Jichun Wu, and Qian Zhang ACS Sustainable Chemistry & Engineering 2021 9 (35), 11635-11651.
You here from time to time from people with rather glib wishful thinking that hydrogen is so called "green" energy because its combustion product is "only" water.
Despite oodles and oodles and oodles and oodles of papers in the literature, and hundreds of thousands of internet posts about "green" hydrogen made with so called "renewable energy," where so called "renewable energy" is defined largely by solar and wind energy, the overwhelming amount of hydrogen produced on this planet is produced from the reformation of dangerous natural gas at high temperatures, the temperatures being provided by burning dangerous natural gas and dumping the dangerous natural gas waste carbon dioxide into the atmosphere without restriction.
That's a fact.
Facts matter.
So called "renewable energy cannot survive without access to dangerous fossil fuels and this paper, which bills itself as an energy storage paper - energy storage is by definition an exercise in wasting energy (unless it recovers energy that would have been wasted anyway) - is no exception.
"DRM" in the context of this paper, is referred to as "dry reforming of methane." Dry reforming is a well known technology in which carbon dioxide is utilized as an oxidant, and in turn is converted to carbon monoxide. Dry reforming is possible with many carbon compounds; the dangerous fossil fuel methane, the chief component of dangerous natural gas just happens to seem cheap, because the real costs will be paid by future generations, about whom the current generation couldn't give a rat's ass.
This paper is, about solar reforming. In these times any appalling and unsustainable practice can be greenwashed simply by sticking the word "solar" on it and assuming most people are too lazy to see through it, generally a good bet for advertisers.
From the text:
Light-driven thermochemical CO2 splitting involves two steps such as the decomposition of appropriate metal oxides at very high temperature (usually above 1600 °C) and the oxidation of the reduced metal oxides by CO2 to produce CO by using very high concentrated solar illumination (e.g., 1500 suns).(13,14) The major challenge is the difficulty of realizing the decomposition of metal oxides with moderate concentrated solar illumination due to the thermodynamic limit of metal oxide decomposition. Photocatalytic CO2 reduction and DRM involves CO2 reduction by electrons and the oxidation of H2O or organic compounds (e.g., CH4 for DRM) by holes on semiconductor photocatalysts. Extensive works have been devoted to improve the fuel production rate (rfuel) and light-to-fuel efficiency (η
I've been hearing all kinds of wonderful stuff about solar thermal technologies my whole adult life by the way, and I'm not by any stretch young. Nevertheless, despite all this cheering, we saw concentrations of 420 ppm of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere this spring, less than ten years after we first saw 400 ppm.
Facts matter.
The authors continue:
Although most of what is written in this paper is disturbing, to me at least, there is some useful science in it, a nice mini-review of sorts of dry reforming.
To wit:
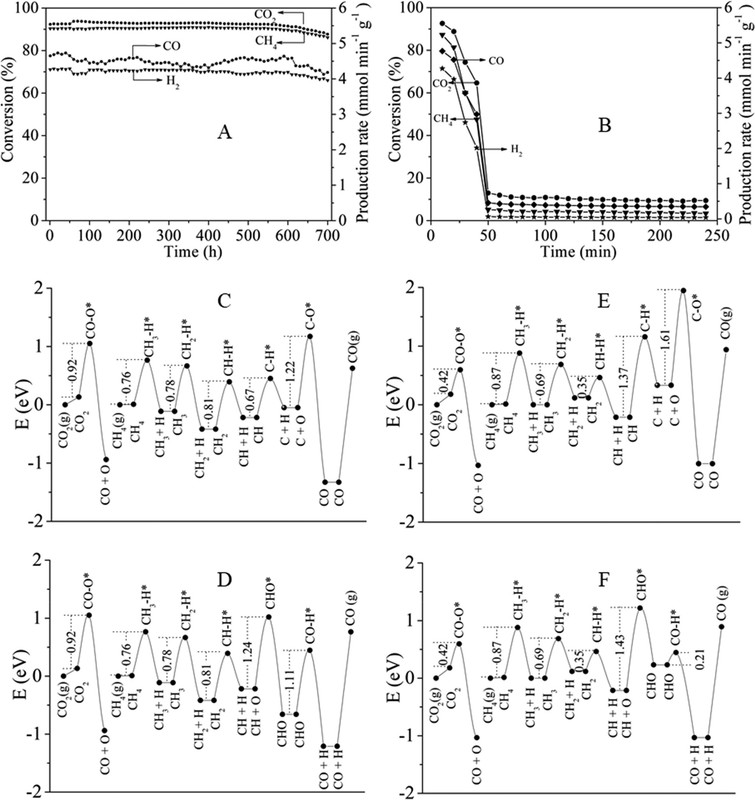
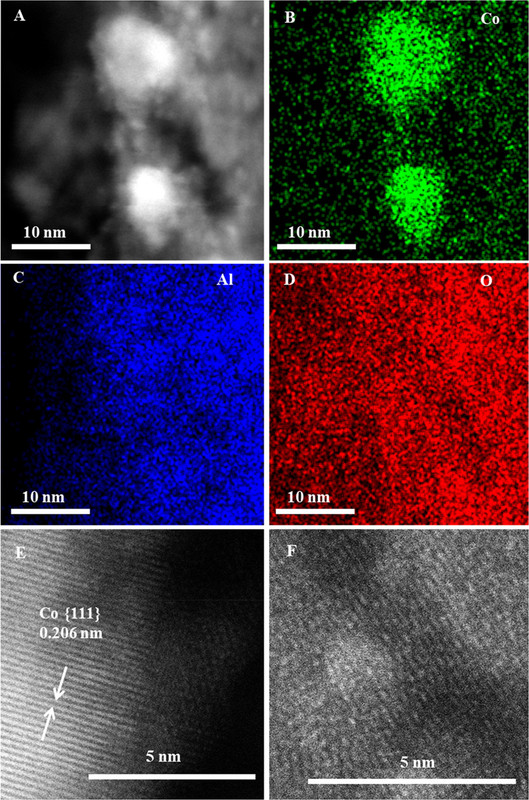
With these ideas in mind, a gas-phase reactor for photothermocatalytic DRM (Figure 1A) was designed.(87) Focused illumination from a 500 W Xe lamp was used to drive the reaction. No additional heater is used besides the Xe lamp. A nanocomposite of Pt nanocrystals (with average particle size of 2.1 nm) partially confined in mesoporous CeO2 nanorods (Pt/CeO2-MNR) was prepared. A known amount of the catalyst was put in a thermal insulation sample holder to reduce heat loss. A feed stream of CH4 and CO2 was continuously fed into the reactor.
Note the Xe lamp. One would think that more than half a century into wishful thinking about how solar energy would save us - many of us having bet the future of humanity on this unproved supposition - that there would be lots and lots and lots of handy solar thermal reactors lying around with which to do experiments. This however, would involve scientists working on grants, some working for Ph.Ds. to be able to work only when the sun is shining brightly and hotly. This would limit the lab time, so xenon lamps are used as a surrogate.
This should tell you something.
Don't worry. Be happy. Let's just look at the pictures from the text and feel all fuzzy inside.
The introductory cartoon:
A picture of the apparatus with the xenon lamp:
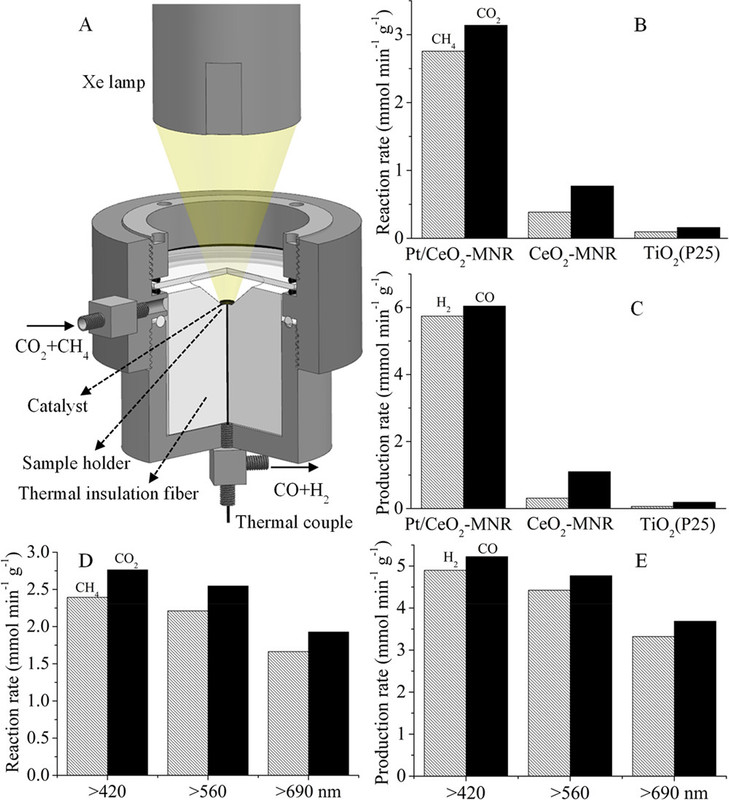
The caption:
Reference 87 is this one: Solar-light-driven CO2 reduction by methane on Pt nanocrystals partially embedded in mesoporous CeO2 nanorods with high light-to-fuel efficiency† (Li et al, : Green Chem., 2018, 20, 2857)
The work was performed in China, and the odds are overwhelming that the Xenon lamp was produced using electricity generated in a coal fired plant.
Nevertheless if you want to do so, and many people do like to read this kind of garbage, you can head over to read rhetoric by a blatantly dishonest bunch of disingenuous horseshit by an anti-nuke telling us how, in China, so called "renewable energy" had defeated the nuclear industry, using all kinds of misleading charts and graphs designed around the assumption you're stupid:
An Exercise in Bad Thinking: Nuclear In China Shows Clear Scalability Winners The barely literate dweeb who wrote this piece and published it in the year we first saw 420 ppm of the dangerous fossil fuel waste carbon dioxide in the atmosphere, less than 10 years after we first saw 400 ppm of the same waste now setting the planet afire, like most anti-nukes, is interested in so called "renewable energy" as an attack on the only climate change tool that actually works, nuclear energy, and couldn't give a rat's ass about dangerous fossil fuels, the use of which is growing, not falling. His name is Michael Bernard, and he wants you to know that he knows Leonardo Di Caprio and that he's willing to consult for you, presumably for a fee.
If you don't know what you're talking about, make stuff up.
His triumphal account includes a picture which I personally find disgusting, of a huge stretch of land covered by soon to be electronic waste, which may be, for a short period of a day, be able to produce as much power as a nuclear plant on less than 15 acres can produce reliably, night and day, 24/7, 365.25 days per year.
Land use changes are second only to the indiscriminate dumping of dangerous fossil fuel waste in driving carbon dioxide concentrations ad climate change.
Feel Free to call on him if you think the laws of thermodynamics should be repealed.

The caption:
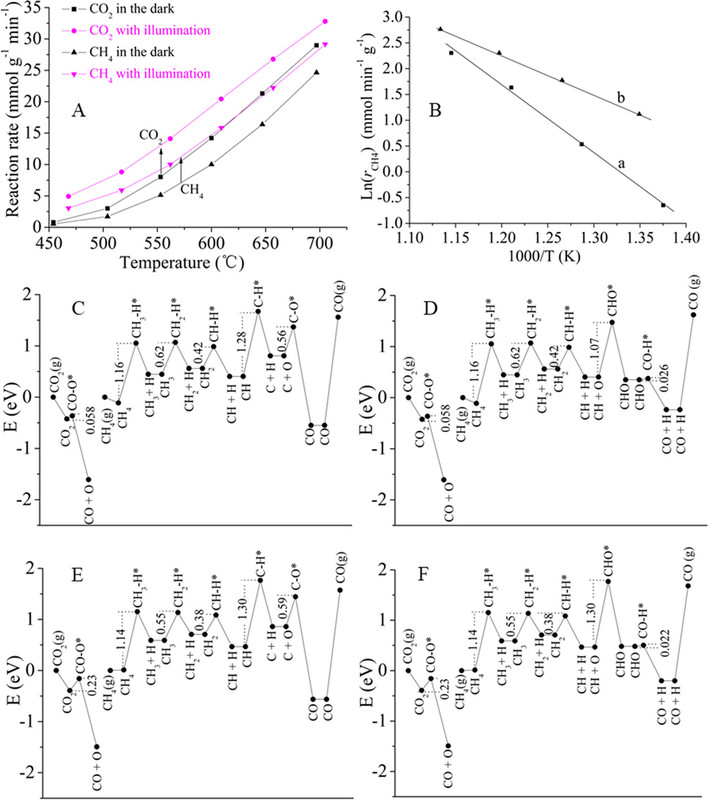
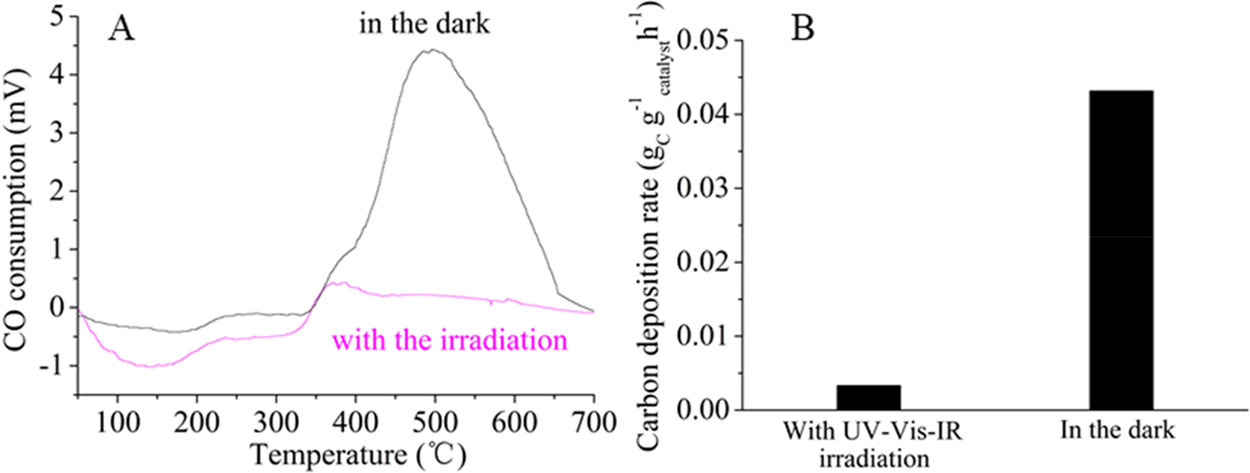
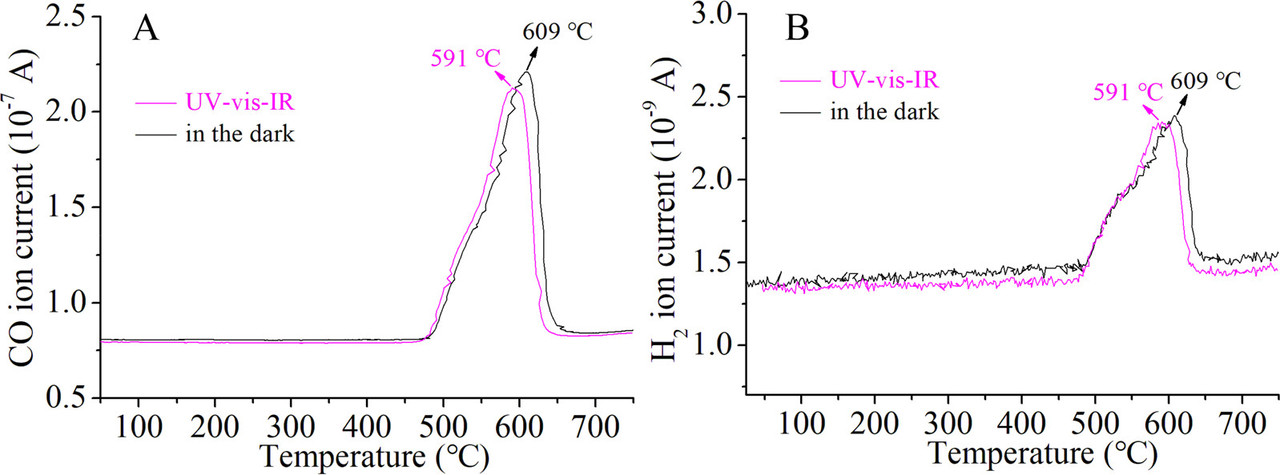
Figure 2. Optical absorption spectra of Pt/CeO2-MNR and CeO2-MNR (A). Time course of production rates for photocatalytic DRM on Pt/CeO2-MNR under UV–vis–IR illumination at near room temperature (B). Schematically illustrated light-driven thermocatalytic DRM on Pt/CeO2-MNR (C). The Teq values of the samples and the sample holder under focused UV–vis–IR illumination (D). Thermocatalytic activity of Pt/CeO2-MNR for DRM in the dark at different temperatures (E and F).(87)
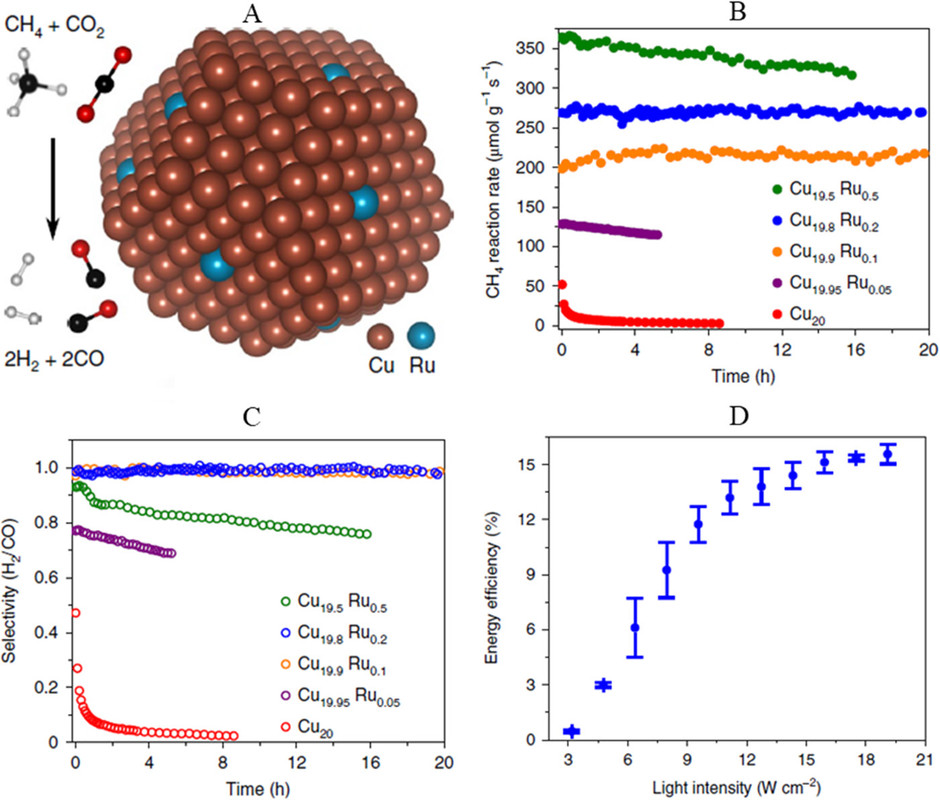
The caption:
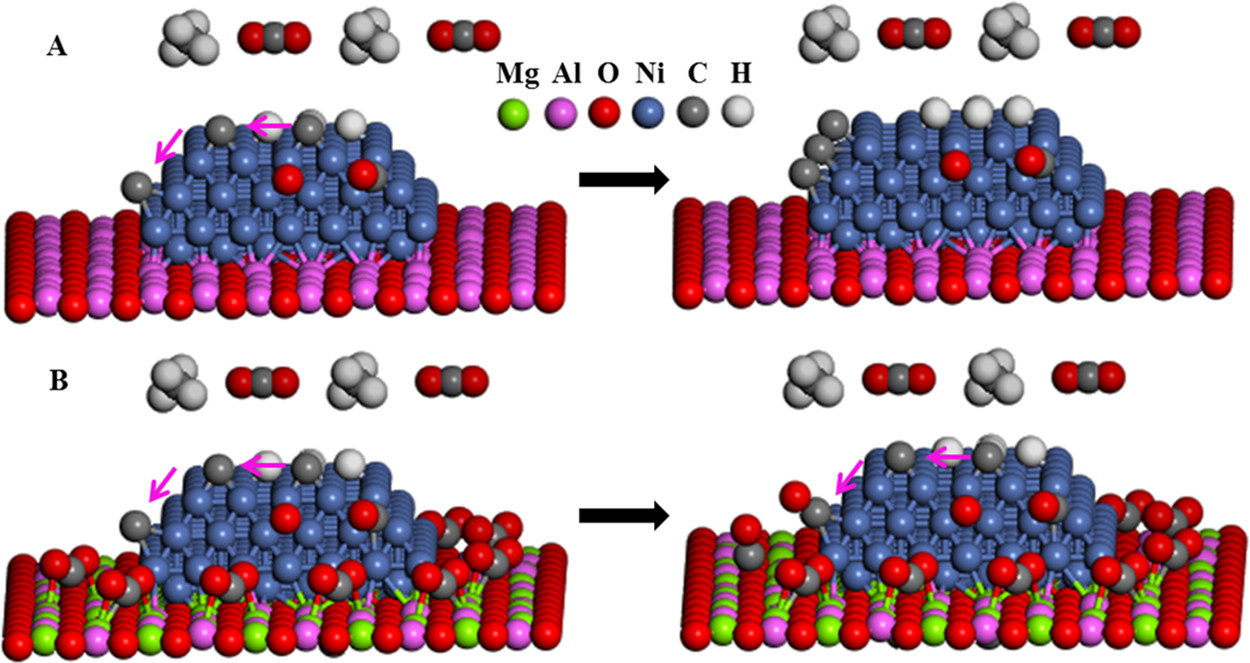
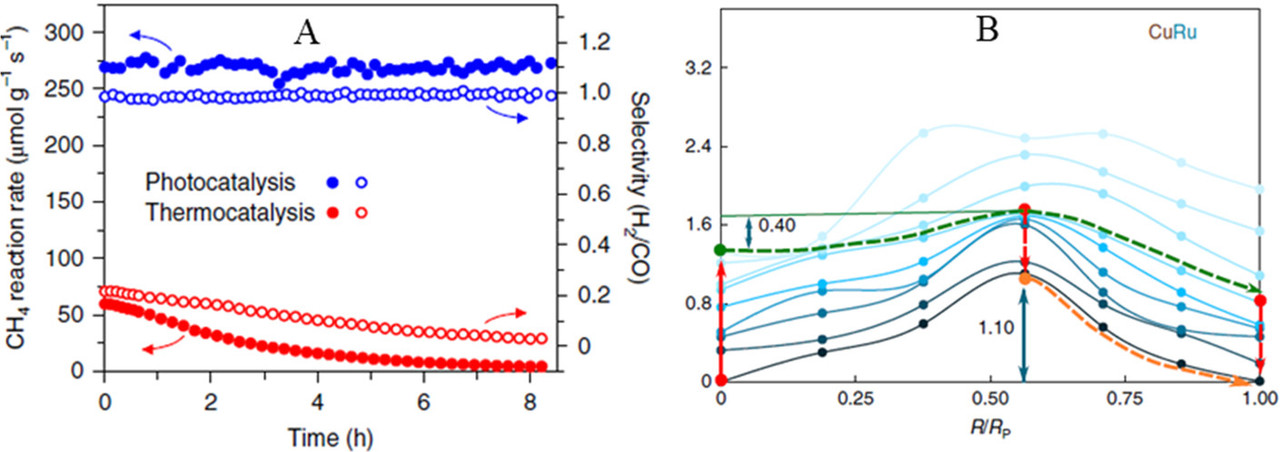
Ruthenium is a relatively rare element, but it can be obtained from used nuclear fuels as it is a fission product.

The caption:
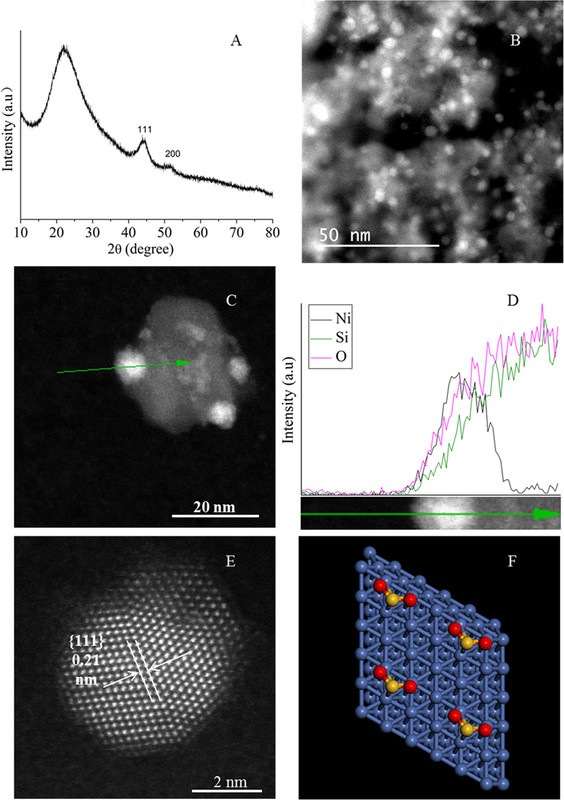
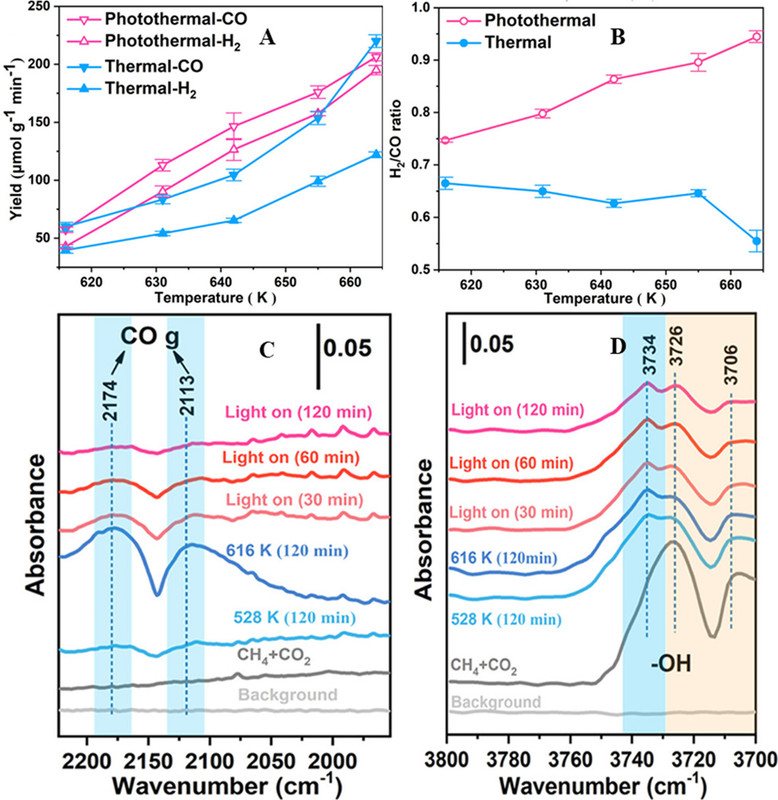
The caption:
The caption:
The caption:
The caption:
The caption:
The caption:
The caption:
The caption:
The caption:
Gallium is a critical element. The world will run out of methane, the sooner the better from my perspective, but the costs of using this methane in our generation will remain with humanity as long as humanity exists.
Have a nice evening.
pbmus
(13,120 posts)‘Methane (CH4): Methane is emitted during the production and transport of coal, natural gas, and oil. Methane emissions also result from livestock and other agricultural practices, land use and by the decay of organic waste in municipal solid waste landfills.’
We will have methane until the end of time…![]()
NNadir
(36,778 posts)...fossil methane, dangerous natural gas. This dominates, by orders of magnitude, the world methane supply.
The background methane, which has little industrial use, can be roughly discerned by looking at the Mauna Loa atmospheric observatory methane pages, Annual Increase in Globally-Averaged Atmospheric Methane
Despite much rhetoric to the contrary, the world is not going to run on cow shit, human shit and landfill gas, although it does, at least in a metaphorical sense, run on horseshit.
The unit in the atmosphere as reported at the NOAA Mauna Loa website is parts per billion. While it is the second most important climate forcing gas, the higher concentrations of carbon dioxide are driving climate change.
The point, despite whatever disingenuous "gotchas" anyone would like to throw is that in order to reform methane using solar energy, one would almost certainly require a concentrated source of it, covering huge stretches of land area for the dubious purpose of reducing carbon dioxide only to dump it again.
I fully concede though, that in theory, we could attach lots of rubber tubes to assholes and collect the methane that otherwise ends up in the atmosphere.
Thanks for the quibble which while accurate has nothing to do with the point whatsoever.
Industrial routes
This diagram shows a method for producing methane sustainably. See: electrolysis, Sabatier reaction

There is little incentive to produce methane industrially. Methane is produced by hydrogenating carbon dioxide through the Sabatier process. Methane is also a side product of the hydrogenation of carbon monoxide in the Fischer–Tropsch process, which is practiced on a large scale to produce longer-chain molecules than methane.
Example of large-scale coal-to-methane gasification is the Great Plains Synfuels plant, started in 1984 in Beulah, North Dakota as a way to develop abundant local resources of low-grade lignite, a resource that is otherwise difficult to transport for its weight, ash content, low calorific value and propensity to spontaneous combustion during storage and transport.
Power to methane is a technology that uses electrical power to produce hydrogen from water by electrolysis and uses the Sabatier reaction to combine hydrogen with carbon dioxide to produce methane. As of 2016, this is mostly under development and not in large-scale use. Theoretically, the process could be used as a buffer for excess and off-peak power generated by highly fluctuating wind turbines and solar arrays. However, as currently very large amounts of natural gas are used in power plants (e.g. CCGT) to produce electric energy, the losses in efficiency are not acceptable.
NNadir
(36,778 posts)Solar cells, for one thing, are not remotely sustainable. Only continuous processes have any chance of being environmentally benign, and it's widely reported that sunlight is not available for half a day on average.
The requirement for heat is a demonstration of the thermodynamic penalty of the process. There is no reference to the source of concentrated CO2 gas.
Electrolysis wastes energy. If solar electrolysis were going to happen on a meaningful scale, it would have happened decades ago; I personally have been hearing this horseshit for at least 40 years, maybe longer. The amount of hydrogen being produced on this planet using dangerous natural gas is higher than at any other point in history. This concept has been rearing it's head in the literature regularly, year after year, decade after decade. After all this talk, where is it?
It is an abuse of language to call that diagram a diagram to produce "sustainable" methane.
I grant that the word "sustainable" is abused often, which is why we hit 420 ppm of carbon dioxide in the planetary atmosphere this spring, because people look at flow charts like this and think they mean something.
Eko
(9,724 posts)gracefully admit you were wrong. Or at least not act like a teenager about it. Not much of a scientist if you cant do that.
NNadir
(36,778 posts)Last edited Sun Sep 12, 2021, 05:06 PM - Edit history (2)
...one might have some idea what scientists are like, how they behave, and what they do.
Of course, I spend a lot of time in scientific journals, as my personal journal here reflects, and, in fact, I'd estimate that more than 50% of my posts are commentaries on scientific articles in scientific journals. I've written so many of these, I've lost count.
If one doesn't know any thing about science, clearly hates science and scientists, one could, I suppose do things like attempt to weakly insult a scientist by following one around to whine about issues of his or her personality.
I have known or met or worked with thousands of scientists in my career, maybe tens of thousands, and I can clearly say that the relationship between producing good science and having a pleasant personality is very weakly correlated, if correlated at all.
I once closely worked with an organic chemist who was excellent at organic synthesis and made many difficult compounds successfully, but was contemptuous of women in the lab to the extent that in spite of his scientific excellence, he had to be fired for being an asshole.
We are seeing this increasingly, particularly in the "me too" era, but also in areas outside of sexual harassment to include racism, fraud, and many instances in which hostile environments are created.
Scientists are also able to express contempt for ignorance without damaging their scientific credibility in any way.
I for instance, find ignorant nitpicking little whiny shits to be annoying, and sometimes openly express my contempt for them in social settings or in social media.
It happens that none of my subordinates in the lab ever experience expressions of contempt from me, but then again, they may not be as inclined to be whiny little shits when speaking with me as much as are some whiny types may be on blogs, as I'm senior management.
Here and elsewhere, I'm just another blogger. I frequently encounter people who know no science, and thus inflate non-events, like say the collapse of an old storage tunnel at a radioactively contaminated site into an event worthy of worldwide attention, this on a planet where 18,500 people die every day because we don't use nuclear energy to its full potential, for one example.
I'm inclined to treat such head up the ass rhetoric with contempt, and sometimes do.
These expressions of contempt, which I consider to be worthy of my sense of what human decency would involve, nonetheless have no bearing on my ability to read, interpret, and utilize scientific information, or my ability to engage in scientific discoveries.
If one were to read the scientific literate diligently and consistently, one would find many examples of scientists who do not "gracefully admit that they were wrong." Perhaps one of the most famous cases of this concerns the Nobel Laureate H.C. Brown, who was definitively wrong for refusing to accept the existence of non-classical carbocations, and despite much experimental evidence produced by oodles of other scientists, proving him to be wrong, never admitted as much.
The Norbornyl Cation Structure (Really) Nobel Laureate Herb Brown was clearly being a dick, loudly and often, but no one would say that he was a poor scientist.
This is hardly the only case by a long stretch. In fact, the majority of the issues of the journals I regularly read, often include at the end of the full journals "comments" on previously published paper, often asserting that the paper was wrong, and only in a low minority of cases does the author of the original paper cheerfully admit to being wrong. Some of these exchanges do border on overt hostility.
One example of a fairly hostile exchange, can be found in the comments and response to comments made by the prominent climate scientist James Hansen and his coauthor, about his famous paper demonstrating that nuclear energy saves lives, a paper I often cite:
Prevented Mortality and Greenhouse Gas Emissions from Historical and Projected Nuclear Power (Pushker A. Kharecha* and James E. Hansen Environ. Sci. Technol., 2013, 47 (9), pp 4889–4895)
The comments and the response to comments are found in this issue: Environmental Science and Technology, June 18, 2013 Volume 47, Issue 12
The fact that Kharecha and Hansen rightly dismissed the comments from the usual anti-nuke idiots did not stop one of those anti-nukes, the marginally educated but frequently published Benjamin Sovacool, from citing his objection comment in a paper he was compelled to retract, this bit of precious nonsense: RETRACTED ARTICLE: Nuclear energy and path dependence in Europe’s ‘Energy union’: coherence or continued divergence?
He's a sloppy little rube in my opinion, completely unqualified to comment on nuclear energy.
Another of the dumb shit anti-nuke commentators, Mark Z. Jacobson, did not gracefully acknowledge criticism of his paper in PNAS that the world could live on 100% so called "renewable energy," a statement that his paper was wrong - which it clearly is - by a consortium of scientists, including a scientist from his own institution publishing in the same journal. Instead, he embarrassed his institution and the larger scientific community by suing the criticizing authors.
JUDGE RULES ‘100% RENEWABLES’ RESEARCHER MUST PAY ATTORNEY FEES FOR HIS DUBIOUS LAWSUIT
Think any dumbass anti-nukes followed him around telling him about how to be a scientist? No. I know of one anti-nuke who used to write here who worshipped this ass.
This aside, scientists, good scientists, outstanding scientists, can and do make mistakes and often 'fess up spontaneously, often apologetically and sometimes cheerfully.
The Search Engine at the American Chemical Society publication site lists 12,290 cases in which the word "correction" is a title word, often the only title word.]
Sometimes scientists do not catch or own up to their mistakes and are, again, called out for them, but not all of these call outs have much meaning; some are trivializing. I made a mistake in using the simple word "methane" above instead of the phrase "industrial scale methane." It was called out, correctly, as a mistake, but it has no bearing at all on the tenor of the OP.
The existence of cow farts and swamp gas has no bearing on the industrial stupidity of reforming methane with solar heat, the point of my post, such a reforming scheme being a dumb idea if ever there was one, which I pointed out.
Whatever.
Over my lifetime, I met lots of people who claimed to respect science and scientists simply, for example, by posting a picture of Albert Einstein somewhere, sometimes in comedic pose, while actually hating science and scientists, mostly out of complete ignorance of what science is and what scientists do as well as the fact that all scientists are, in fact, human beings, with all the strengths and weaknesses that being a member of the human race implies.
As a scientist, I know of at least one case where a dumb guy follows my posts around on a blog nitpicking and whining because I called him out on stupid and irrational fears of radioactivity. I consider this type of fear and ignorance to be as toxic as anti-vax rhetoric, since given Hansen's paper cited above, and given the Lancet Global Burden of Disease Study papers detailing the number of deaths from air pollution - the most recent figure being on the order of 18,500 people per day, more than Covid killed worldwide on its worst day - since like anti-vax rhetoric, it kills people. It's been doing so for decades.
I clearly have zero respect for the guy offering up such trivial radiation paranoia.
I would advise such a person to get a life, to get past the little wound to their questionable ego, but somehow I think it's not likely.
Eko
(9,724 posts)Not only do you strawman more than anyone I know but you are a very unpleasant person. What you call a stupid and irrational fear of radioactivity was just me pointing out that nuclear energy also had it hazards when referring to solar vs nuclear where you said solar was more dangerous. That's it. From that you have moved on to I am anti-nuclear when I have said multiple times I think we need nuclear as well as solar, wind, and whatever we can do to get off the energy sources that are killing our planet. And anyone who knows me knows I have the utmost respect for science and scientists and no where at any time have I ever said anything contrary. But now I hate them according to you, Why? because you got your fee fees hurt. But ya got to strawman anyone who doesnt agree with you or even calls out your mistakes and just be a little sh*t about it. I honestly dont think you are capable of seeing your behavior in any other light but positive but I will try to show you once again. You know you are talking about me with this "like say the collapse of an old storage tunnel at a radioactively contaminated site into an event worthy of worldwide attention, this on a planet where 18,500 people die every day because we don't use nuclear energy to its full potential, for one example. " First, you cant even engage in any honest way by admitting that you are talking about me and second that is not what I did at all. No where did I anywhere say that it was an event worthy of worldwide attention, it was just to point out that nuclear also has its hazards. If you dont remember this here is the link to it to refresh your memory. https://www.democraticunderground.com/1127110309#post16