Environment & Energy
Showing Original Post only (View all)The Removal of Some Radionuclides from the Nuclear Weapon Waste Tanks at Hanford w/Titanate IE. [View all]
During the Second World War, and then during the "Cold War" the United States and, after 1945, the former Soviet Union raced to make weapons grade plutonium at a prodigious rate. Weapons grade plutonium has specifications for isotopic purity requiring that require being as close to monoisotopic Pu-239 as is possible. Since the capture to fission ratio of Pu-239 in a neutron flux is not zero, this means that the irradiation periods in the fuel rods needed to be relatively short, with the result that the plutonium in the fuel be fairly dilute. As the concentration of plutonium reaches a certain level, the probability of a neutron being absorbed by Pu-239 to give Pu-240 increases, eventually beyond a point that exceeds the specifications for nuclear weapons. (Nuclear weapons with too much Pu-240 "fizzle." ) Given the methods utilized to isolate plutonium, first the Bismuth Phosphate process, and then the solvent extraction based Purex process, coupled with the unusually dilute concentrations of plutonium in the fuel, large amounts of chemical waste, containing significant radioactive species was generated.
These chemical and radiological wastes were at first dumped in open trenches, but ultimately were added to a rather large series of now famous tank farms, most of which are way beyond their design life. Some are known to be leaking, others are expected to leak.
These tanks are the topic of discussion in the paper I will discuss in this post: Evaluation of Load Behavior for Select Analytes in Hanford Tank Waste, Amy M. Westesen, Emily L. Campbell, Sandra K. Fiskum, and Reid A. Peterson, Industrial & Engineering Chemistry Research 2022 61 (32), 11691-11698
The Hanford tanks, and the Hanford site in general, are favorite topics for discussion by antinukes. They live under the assumption that any exposure to radioactive materials is a vast tragedy, outweighing for instance, the hundreds of millions of people who have died from air pollution since, say, 1986. They almost never report a death toll associated with exposure to radionuclides (in non-medical settings), but nonetheless they get very excited by any such exposure; lots of coal has been burned to power computers and website servers devoted to often hysterical discussions of such exposure. I have chosen the year 1986 because herein I will discuss the properties of wastes found in the 241-AP Hanford tanks in the 200 area of the Hanford Nuclear Reservation, which accepted waste up until 1986, thirty six years ago. These tanks are "double shell" tanks; they had a design life of 50 years as shown in the following table:

RPP-RPT-55982, Rev. 0 241-AN Tank Farm Construction Extent of Condition Review for Tank Integrity
It is not a coincidence, by the way, that the tanks fell out of use in 1986. 1986 was, of course, the year the Chernobyl reactor exploded. The N-reactor (like the Chernobyl and other RMBK reactors) was a potential dual use reactor, for both producing electricial power and weapons grade plutonium. It was the only nuclear reactor in the United States that had a similar design to Chernobyl, inasmuch as it was graphite moderated and lacked a containment structure. Unlike Chernobyl - but certainly not other RBMK reactors in the former Soviet Union - the N-reactor was not potentially dual use; it was actually dual use. Almost all of the weapons grade plutonium in US nuclear weapons produced after 1963 was produced in the N-reactor, although some weapons grade plutonium was probably recycled from older decommissioned weapons. (Understanding the long term stability of metallic plutonium in the delta phase was the primary goal of underground nuclear testing that lasted almost to the end of the 20th century.)
The N-reactor was shut in 1987 to avoid "another Chernobyl" although the fact of the matter that the explosion of the Chernobyl reactor required really bad management to fail as it did.
By the way, the Soviet equivalent of Hanford was Mayak, where rather than dump the waste in double shell tanks (or even single shell tanks), the waste was dumped in a lake. Unsurprisingly, the lake is in bad shape.
I discussed Hanford and Mayak in a rather long and lugubrious post here: 828 Underground Nuclear Tests, Plutonium Migration in Nevada, Dunning, Kruger, Strawmen, and Tunnels Some information about the residual plutonium from these weapons tests in Nevada geological formations is also included in this long post.
Vast sums of money have been spent "cleaning up" Hanford. To my mind - I'm sure I'm largely a dissident here - I don't necessarily approve of the expenditure, at least most of it (see below), since I don't believe it will save very many lives, since very few lives are seriously at risk. I wonder what the cost will be per "life saved" - if any lives are saved, as opposed to the cost of cleaning up fecal wastes that are uncontained for well over a billion human beings who lack [iany kind of improved sanitation].
It is probably true that much of the expenditure is wasted, a point I made in the afore referenced post when comparing the risk of 4000 truckloads of cement with the risk that someone someday somewhere might eat some Hanford technetium.
There is, despite scant proof of it, that the Linear No Threshold Model "LNT" is a canon - or, in my view, a "cannon" since the assumption's strict regulatory application almost certainly kills people in vast numbers - of irrefutable truth. We will spend almost unlimited amounts of money to prevent a single person dying from radiation exposure related to nuclear fission (but not x-rays or other medical imaging and treatment modalities), while not caring at all about who will die today from exposure from dangerous fossil fuel waste, never mind fecal waste. (Dangerous fossil fuel waste kills about 18,000 people per day.) We simply did not have the molecular biology tools in the 1950's that we have today when the assumption was put forward, nor did we have unintentionally formed sites like Chernobyl, or intentionally formed (albeit for a different purpose than to collect data) like Hanford, or Savannah River in South Carolina. There is considerable evidence, some from the application of modern molecular biological tools, as well as examinations of the original, perhaps dubious, experiments conducted ¾ of a century ago to formulate it, that the “LNT” is highly questionable, and yet it informs our regulatory, social, and (to an overwhelming extent) public perception.
In fact, the only very long term evidence we have of the behavior on actinides and fission products in geologic settings comes from analysis of the Oklo reactors which operated in Gabon nearly two billion years ago. I made reference to the natural nuclear reactors in the post about 828 nuclear tests:
Fission product retention in the Oklo natural fission reactors (David Curtis, Timothy Benjamin, Alexander Gancarz, Robert Loss, Kevin Rosman, John DeLaeter, James E. Delmore, William J. Maeck, Applied Geochemistry, Volume 4, Issue 1, 1989, Pages 49-62)
Although critical masses are very different for oxides than they are for metals, and very different in the presence or absence of water - the cyclical nature of the Oklo reactors was tied to the fact that the water that moderated neutrons and started the reactors boiled, shutting them down until they cooled enough to resume criticality - one may wonder, naively at least, whether it is possible for similar reactors to form at the Nevada National (In)security Site. This, in turn, will depend on the ability of plutonium to migrate as uranium did billions of years ago.
Don't worry; be happy. (I think it very, very, very, very unlikely.)
In situ plutonium was generated at the natural Oklo reactors just as it is in modern anthropogenic nuclear reactors, whether the reactors are constructed for warlike purposes (where the plutonium production is for the more environmentally impactful weapons grade material which must be synthesized in low concentrations in the fuel) or for saving lives from air pollution in commercial reactors. At Oklo, the evidence is that the plutonium was retained in apatite, a mineral also found in synthetic bone and as a result did not migrate very far: Isotopic evidence for trapped fissiogenic REE and nucleogenic Pu in apatite and Pb evolution at the Oklo natural reactor (Kenji Horie, Hiroshi Hidaka, François Gauthier-Lafaye Geochimica et Cosmochimica Acta, Volume 68, Issue 1, 2004, Pages 115-125).
A fairly sophisticated reactor physics analysis of the Oklo natural reactors may be found here: Criticality of the reaction zone 9 of Oklo reactors revisited (K. Mohamed Cherif, A. Seghour, F.Z. Dehimi, Applied Radiation and Isotopes, Volume 149, 2019, )Pages 165-173)
A similar discussion may be found here: R.T. Ibekwe, C.M. Cooling, A.J. Trainer, M.D. Eaton, Modeling the short-term and long-term behaviour of the Oklo natural nuclear reactor phenomenon, Progress in Nuclear Energy, Volume 118, 2020.
Some of the Hanford waste tanks are leaking, and others may or are even likely to leak, and some of the waste, particularly in the early years of its operation, was dumped in open trenches at Hanford, and to the extent these are migrating, albeit in a drier climate than that at Oklo billions of years ago, they offer experimental insight to the geology of radionuclides.
A quick caveat: Many people still think that the appropriate way to dispose of so called "nuclear waste" is to bury it in geological formations such as that proposed, and then abandoned, at Yucca Mountain in Nevada. The Oklo reactors were and probably still are discussed as a justification of the claim that radionuclides will almost certainly not cause cancer putative ranchers living in Nevada, were Yucca Mountain used, in the 25th century if suddenly Nevada is transformed into a tropical rain forest. (It would be an interesting and ethical world if we cared as much about the 18,000 people who will die today from air pollution as we do about this fictional 25th century Nevada rain forest rancher.) I do not approve, personally, of geological disposal of used nuclear fuel. Unlike most advocates of nuclear energy, I am rather happy that Yucca Mountain didn't happen. I believe that nearly every component of used nuclear fuel has value and should be put to use to address otherwise intractable problems. Some of these components are highly radioactive, and will remain so for long periods, so much the better, as the environmental problems they can be used to address, particularly in the case of wide spread chemical pollution of the air, seas, land, and fresh water resources that we face - and all future generations will face - that these products might address will take a long time to ameliorate. My view is that we need more fission products and higher actinides, not fewer.
I have argued and will continue to argue that the solar energy fantasy has proved useless to address climate change, that the trillions of dollars invested into research into developing the technology, manufacturing its components, constructing its infrastructure and connecting all these components together has proved to be a expensive and extremely wasteful endeavor that has done little to save the world now in flames. It failed, is failing and will continue to fail. It's no longer an expensive joke. The specter of climate induced famine is before us.
Considerable amounts of high level radionuclides were removed from the Hanford tanks many years ago. Much of the US industrial and research supply of cesium-137 originated from the wastes isolated from Hanford between 1967 and 1979, with additional purification lasting to 1984. I discussed the procedure utilized for this isolation quite some time ago in this space: 16 Years of (Radioactive) Cesium Recovery Processing at Hanford's B Plant.
The half-life of cesium-137 is 30.08 years. From the radioactive decay law, this means that about 58.3% of this isolated cesium isolated in 1984 has decayed without killing anyone. The balance remains radioactive and useful. (The Goiana event in which four people died and several hundred were contaminated when a cesium-137 source abandoned at a closed medical facility was stolen and opened is thought to have originated from cesium obtained from Oak Ridge National Laboratory by an Italian company.) By contrast, around 71.8% of the cesium-137 isolated from the Hanford tanks in 1967 has decayed since isolated and is thus less useful.
The methods described in the "16 years" report needed to be changed because several of the resins used ultimately failed after some time in use. The full report can be accessed from my 2018 post for anyone interested in the separation science.
The resins used back then were organic resins, and they worked well enough. The paper under discussion in this post uses inorganic resins. I suspect that unfortunately the purpose of these inorganic resins is to put the cesium in a "waste form" rather than to recover it for use, but all the same, it's an interesting paper.
From the introduction:
The engineered sodium form of CST, currently manufactured by Honeywell UOP as IONSIV R9140-B, is a promising ion exchanger for removal of Cs+ due to the structural size of (TiO4) SiO4 tunnels that exist within the material. These tunnels have an ideal size for selective exchange of Cs+ and Sr2+, making these exchangers one of the most promising materials for their removal. (12−14) In addition, the radioactive ions trapped in the inorganic material can be directly immobilized to a ceramic or glass waste form for final disposal, making this material a top choice for Cs removal in defense nuclear waste at the Department of Energy (DOE) Hanford Site.
The Hanford nuclear reservation in Eastern Washington has 56 million gallons of radioactive tank waste remaining from World War II and Cold War production of the nation’s plutonium for its nuclear weapons program. The waste, currently stored in underground tanks, is a chemically complex combination of liquid (supernate), sludge, and salt cake. DOE is tasked with disposing of the highly radioactive waste by vitrification at the Hanford Waste Treatment and Immobilization Plant to immobilize the waste for long-term storage. DOE is working to jump-start treatment of the supernate fraction of waste in 2022 through implementation of the Tank Side Cesium Removal (TSCR) system. TSCR processes the liquid portion of the waste and removes 99.9% of 137Cs, allowing the waste to be contact handled and decreasing the radiation hazard to workers and the environment. The disposition pathway for the spent CST columns has yet to be determined and will depend heavily on the characterization of what has sorbed onto CST. Potential options include vitrifying or grouting the spent CST, which would require characterization for the formulation of additives needed for this disposition.
Pacific Northwest National Laboratory (PNNL) was contracted to test Cs removal with CST in supernates collected from Hanford tanks 241-AP-105, 241-AP-107, and 241-AW-102 (hereafter AP-105, AP-107, and AW-102, respectively) at small scale (10 mL CST beds) under prototypic plant operating conditions. Reported herein are the characterization and comparative ion exchange load behavior of Cs, along with other selected analytes, onto CST from Hanford tank waste. Decontamination factors (DFs) for the uptake of Al, Ca, Pb, Np, Pu, U, and Sr were determined along with removal efficiency by CST. This characterization will help improve understanding of the behavior of CST and assist in identifying potential disposition pathways as well as assessing removal capabilities of CST for other components...
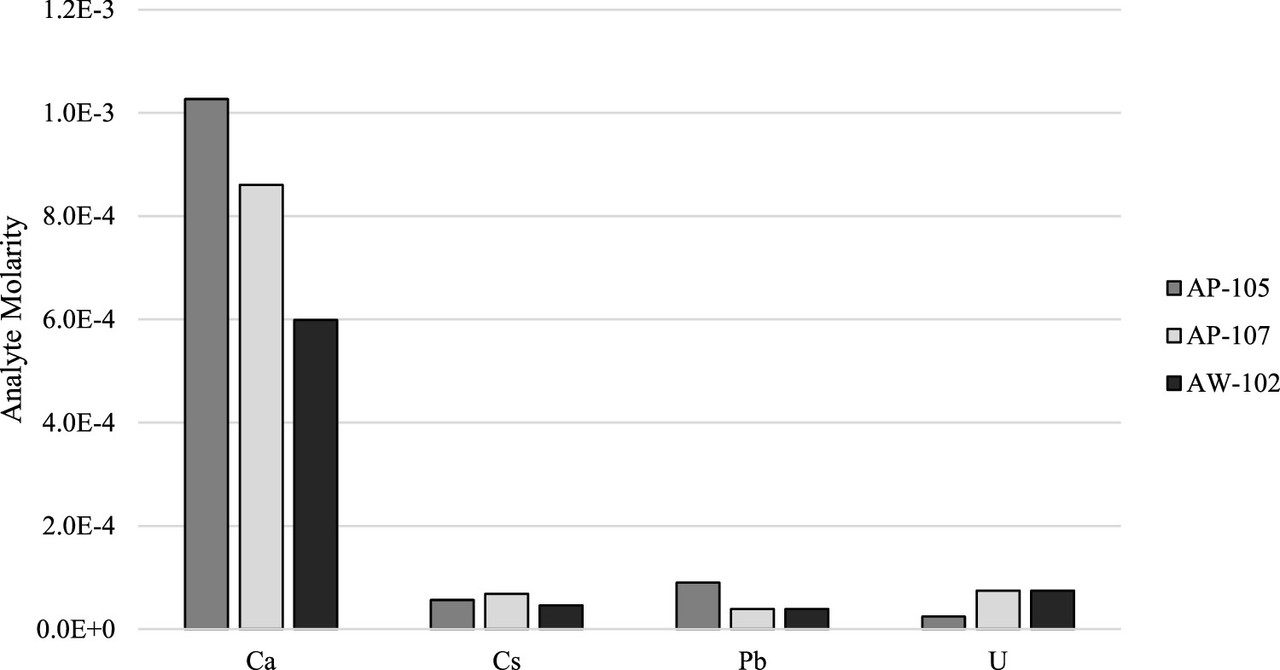
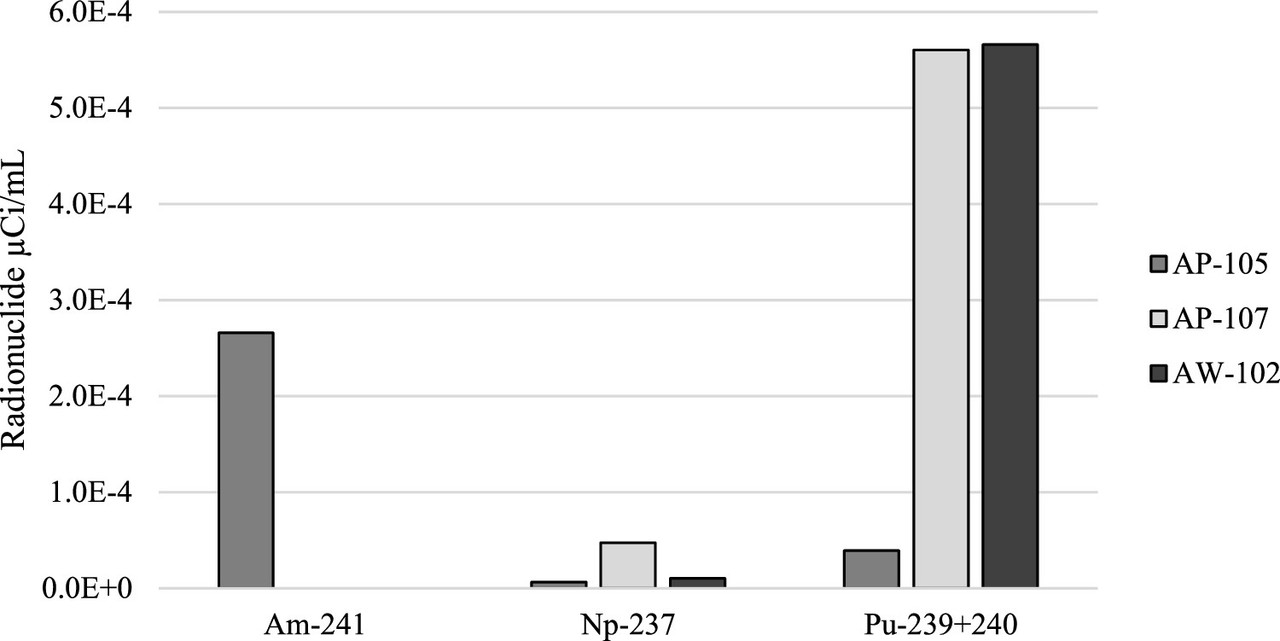
The interesting composition of the tank supernatants can be found in the supplemental information of the paper, which is free to access, Table S2.
The difficulty of this composition as I see it is the large amounts of potassium and sodium in the mixture, congeners of cesium, as well as calcium, the congener of strontium. (It is important to note that the units are different between the non-radioactive species and the radioactive species, respectively units of molarity and units of microcuries. The non-radioactive species dominate the waste. For example, the concentration of Cs-137 is given as 113 µCi/mL in tank AP105. Given the specific activity of Cs-137, the concentration can be shown to be 10 millionths molar. The concentration of sodium is roughly 600,000 times higher than that of cesium. Thus the separation factor is very challenging.
Nonetheless, the authors claim some success.
Graphics from the paper:
The caption:
The caption:
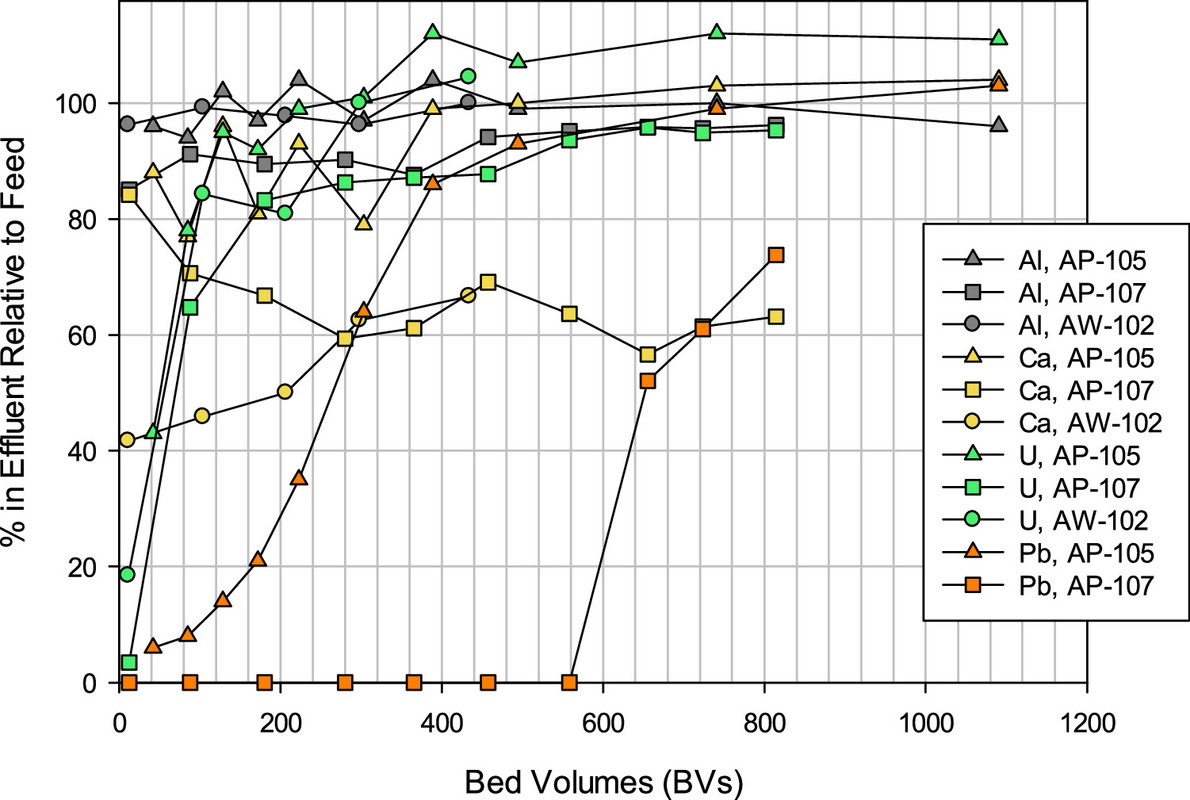
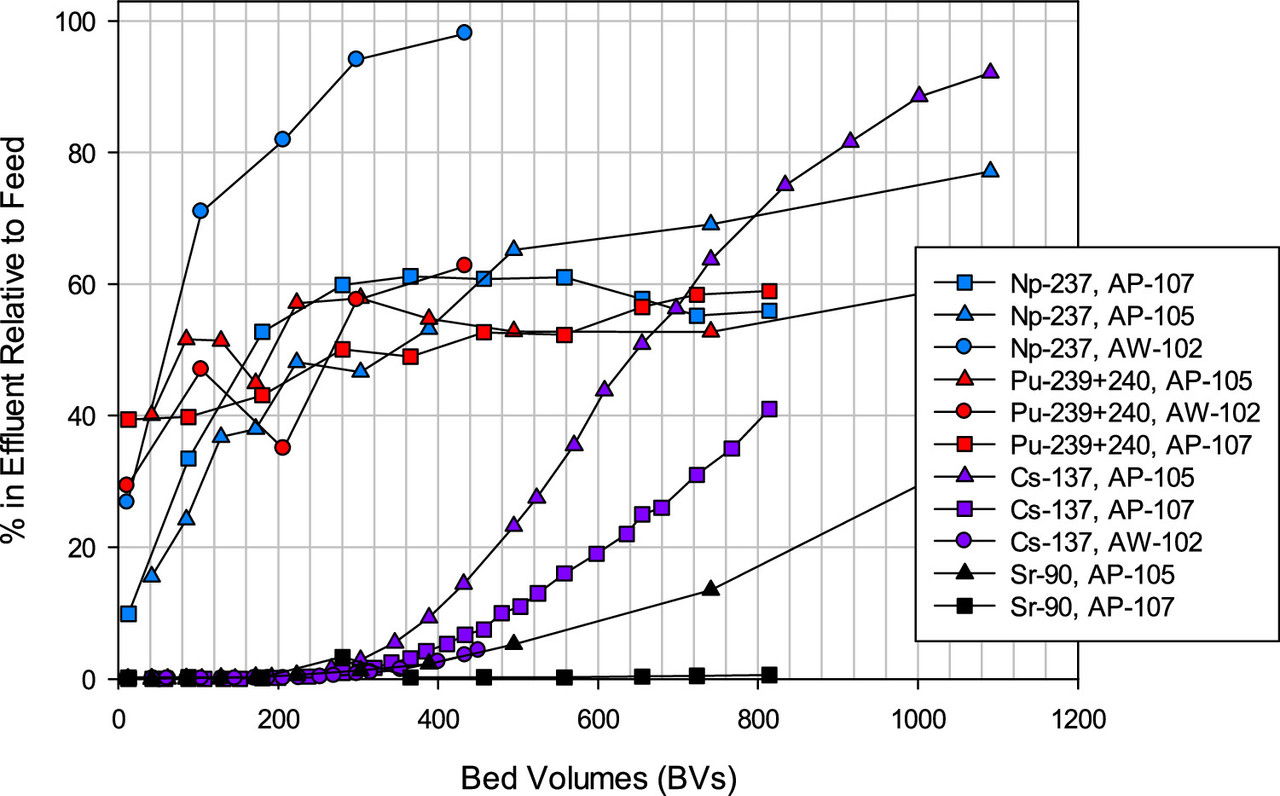
A feel for the separation factors for various elements in the tanks. (Note the different profiles for different tanks, reflecting to be sure, chemical differences.)
The caption:
The caption:
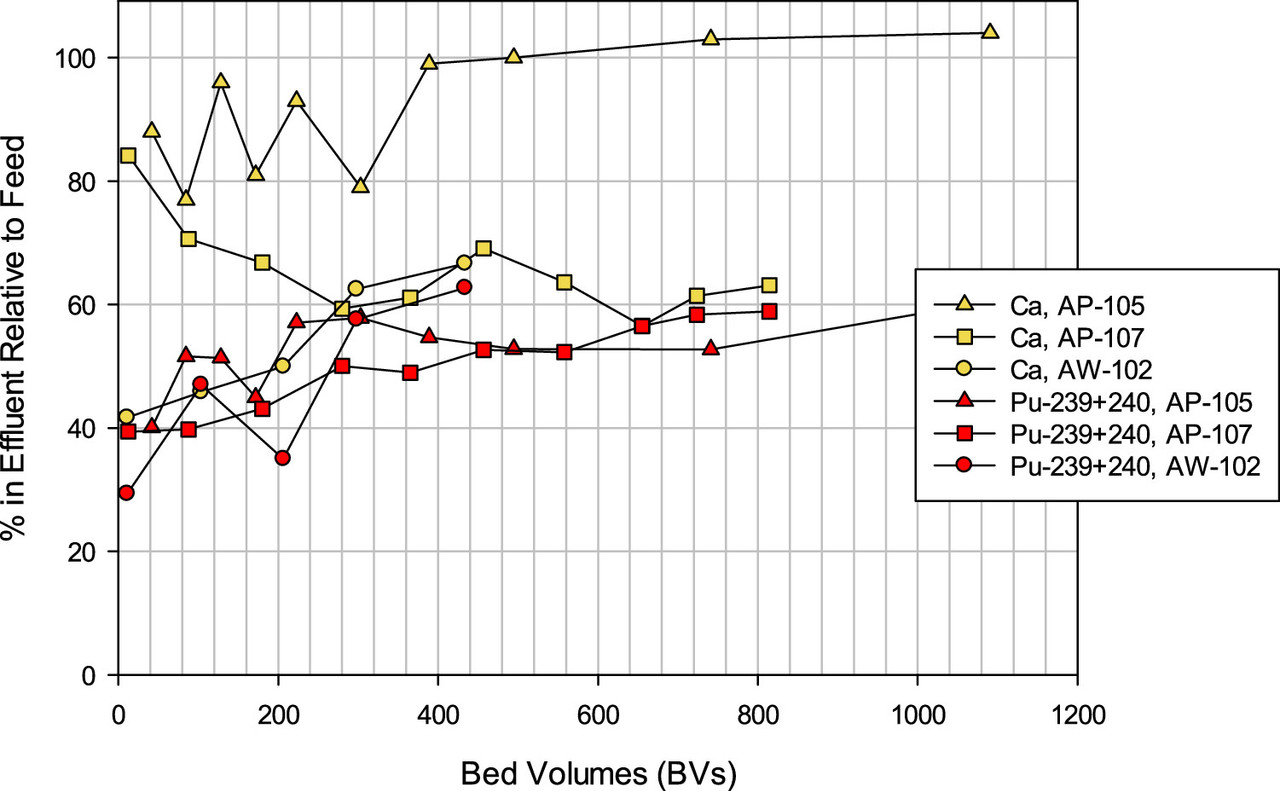
The caption:
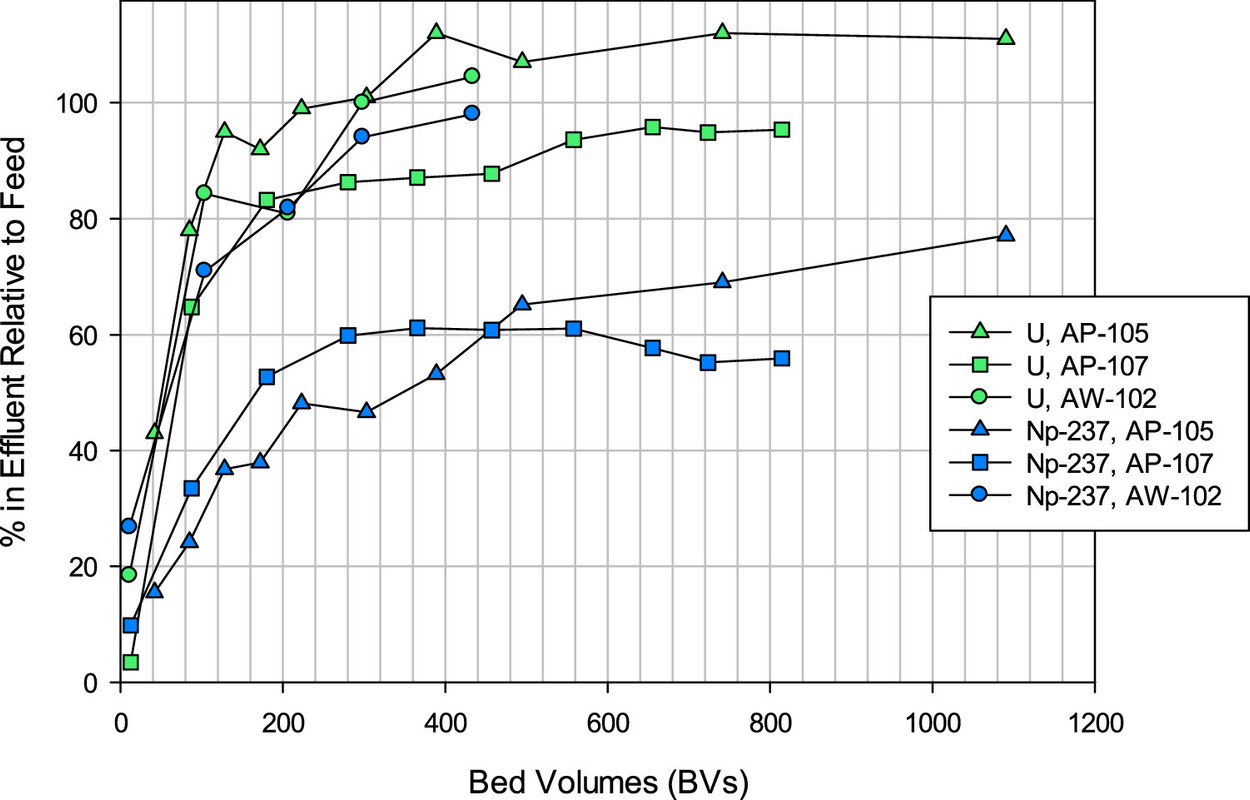
The caption:
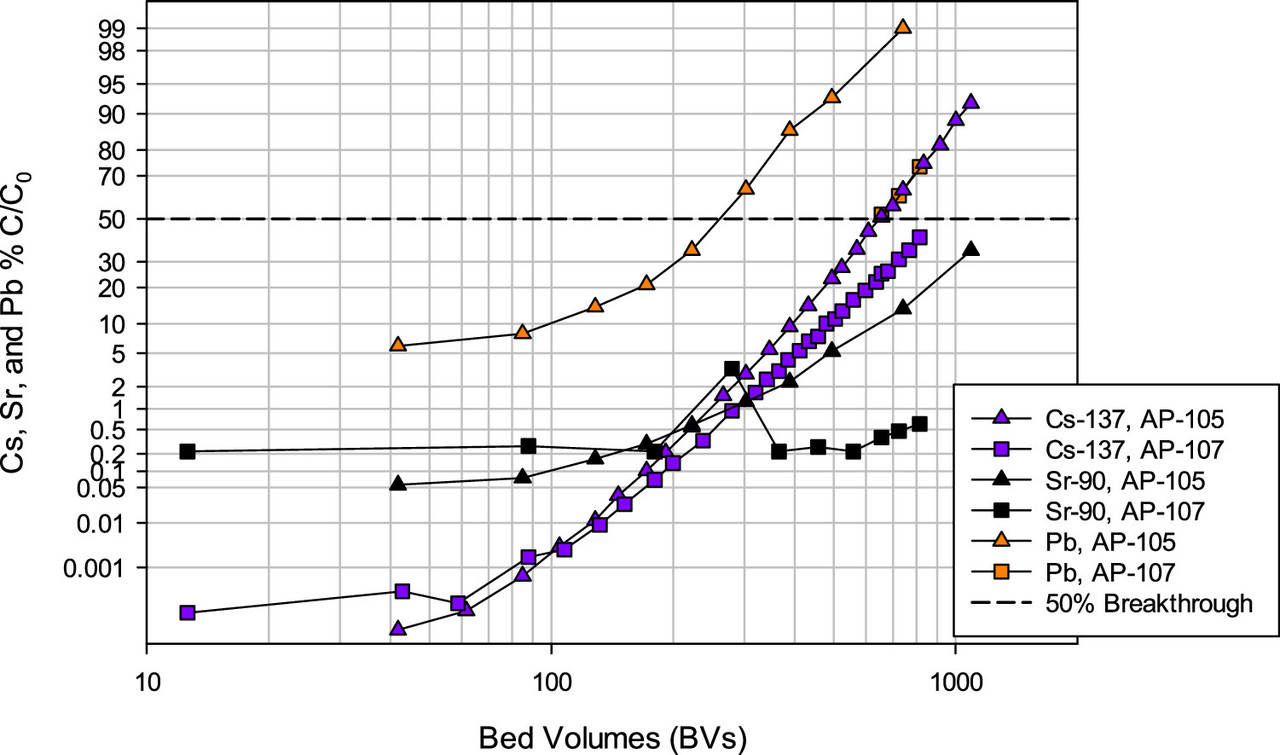
The caption:
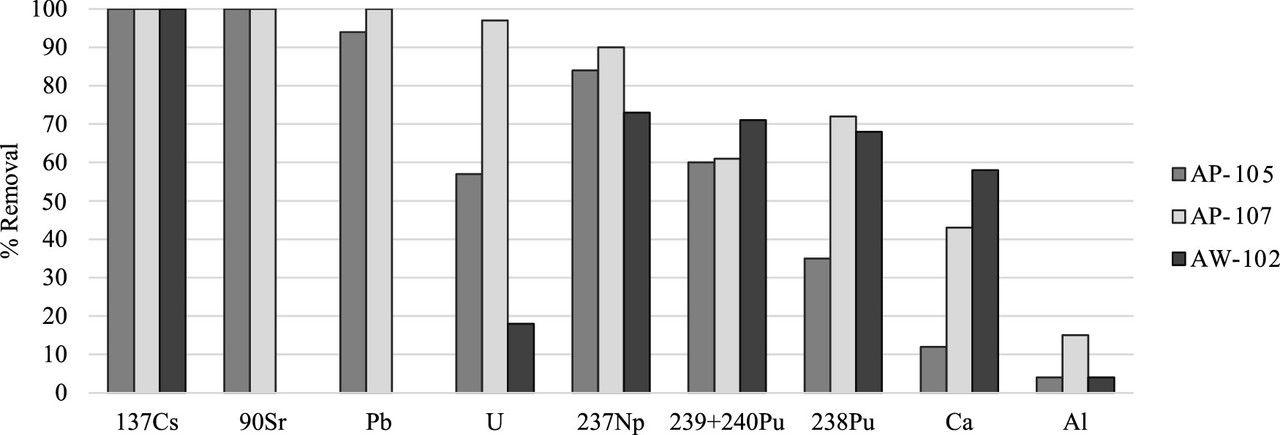
The caption:
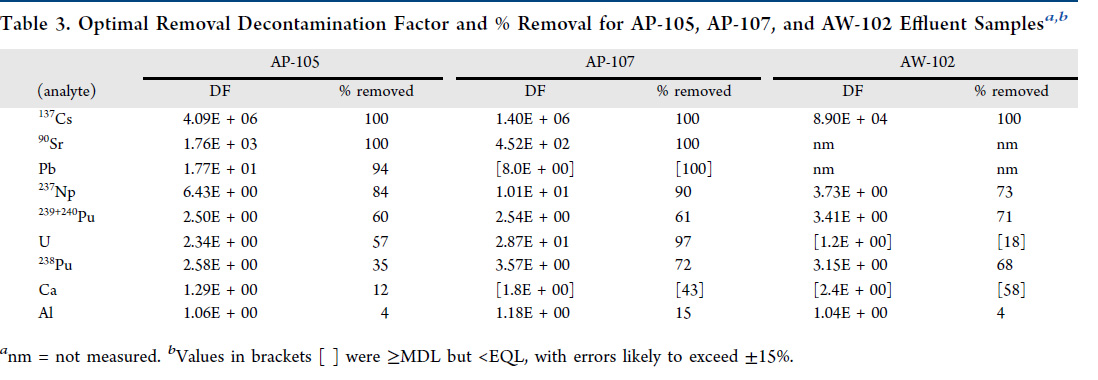
A table from the paper indicates the decontamination factors, rather spectacular in my opinion for cesium and strontium.
From the conclusion of the paper:
Possible drivers for differing analyte recoveries could be variations in process volume leading to differing amounts of analyte recovery in the effluent, changes in analyte concentrations specific to each tank waste feed, and likely complex ion differences of soluble analytes leading to changes in CST uptake. Understanding the behavior of CST and characterization post processing tank waste will assist in determining potential disposition pathways and removal capabilities of CST.
Again, the goal here is not recovery, but rather disposal, which I personally find unfortunate. Over many years of thinking about radiocesium, I have convinced myself that it is a very valuable material with many interesting uses, particularly in ameliorating, in continuous flow systems, some otherwise nearly intractable environmental problems. None of what I think about this, of course, will matter, but I hope I will be able to share these ideas with my son before I die, as he is very likely to be better situated to bring these ideas to fruition than I will ever be.
Cesium titanates are very insoluble, and it is difficult to imagine cesium leaching from a titanate waste form, never mind a titanosilicate. Although cesium is often thought to be a highly soluble and mobile element, and in many chemical forms it is very much so, the world supply of cesium to make cesium formate for the drilling of dangerous fossil fuels because so called "renewable energy" has proved useless to address the increasing use of them, never mind eliminating them, is obtained from minerals mined in Canada, primarily pollucite, an aluminosilicate. These minerals are well over 2.5 billion years old, and isolating cesium (and rubidium) from them is not especially easy.
(cf. Petr Černý, David K. Teertstra, Ron Chapman, Julie B. Selway, Frank C. Hawthorne, Karen Ferreira, Leonard E. Chackowsky, Xian-Jue Wang, Robert E. Meintzer; EXTREME FRACTIONATION AND DEFORMATION OF THE LEUCOGRANITE – PEGMATITE SUITE AT RED CROSS LAKE, MANITOBA, CANADA. IV. MINERALOGY. The Canadian Mineralogist 2012;; 50 (6): 1839–1875.)
Mineralized cesium (and rubidium) can be and is, geologically, extremely stable.
None of these facts will, of course, preclude the kind of assholes who, without ever opening a book, from declaring with monumental ignorance that "nobody knows what to do with..." (what they call) "...nuclear waste."
Nevertheless, it is almost impossible that cesium isolated in this fashion from the Hanford tanks will ever kill anyone, no matter where it's taken, put or stored, certainly nowhere near, over centuries, comparable to the number of people who will die from dangerous fossil fuel waste in the next hour and every hour afterwards, 750 people per hour approximately, 18,000 every damned day, about 7 million people per year and that does NOT include deaths from climate change, but only people killed by the chemical toxins in air pollutants. Climate deaths, including but hardly limited to likely rising famine are a whole other category.
But beyond that, for reasons I alluded to in the "828 nuclear tests" posts, it is probably very unlikely that the leaking tanks from the weapons plant will kill very many people, if any. The situation is very likely to be very much like the Oklo situation nearly 2 billion years ago, with small dilute amounts of radioactivity leaching away in subtoxic concentrations. The vast sums of money to satisfy the dark, and frankly illiterate fantasies of anti-nukes are probably thus all wasted, and history will not look kindly on them.
I know these statements fly in the face of "conventional wisdom," but in these times of rising insanity, "conventional wisdom" is something of an oxymoron, not at all to be trusted, as no "wisdom" is involved.
Have a pleasant day tomorrow.