Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Environment & Energy
Showing Original Post only (View all)All Together Now! Microplastic Pollution, Ocean Acidification, Halogenated Methanes, Ozone Collapse... Delicious! [View all]
Last edited Sun Jun 29, 2025, 09:24 AM - Edit history (1)
Here's a charming paper I came across this morning: Decreased Dimethylsulfide and Increased Polybrominated Methanes: Potential Climate Effects of Microplastic Pollution in Acidified Ocean Qian-Yao Ma, Ya-Wen Zou, Jun-Qi Yang, and Gui-Peng Yang Environmental Science & Technology 2025 59 (24), 12145-12157.
Synergies, you have to love them.
From the text:
Plastic pollution and ocean acidification (OA) are widely recognized as significant existential threats to marine ecosystems. Each year, an estimated 4.8 to 12.7 million tons of plastic debris are introduced into the oceans, where they undergo degradation into tiny fragments or fibers, termed microplastic (MP, particle size less than5 mm) through ultraviolet irradiation, mechanical abrasion, and biological breakdown. (1) Current estimates suggest that between 14,400 and 236,000 tons of MP float on the ocean surface, (2) with projections indicating that by 2100, oceanic MP concentration could be 50 times higher than today. (3) Concurrently, excessive CO2 emissions from anthropogenic activities have resulted in significant changes in the global marine environment, including warming, acidification, and deoxygenation. (4) The oceans absorb over a million tons of atmospheric CO2 per hour, leading to a decrease in global ocean pH by about 0.0166 units per decade. (5) Since the preindustrial era, the pH of surface seawater has decreased by approximately 0.1 units. (4) According to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC), under the SSP5-8.5 scenario─which assumes high economic growth, elevated emissions, and minimal climate mitigation efforts─the mean pH of surface oceans is projected to decline by 0.2 and 0.4 units by 2050 and 2100, respectively, relative to the 1995–2014 baseline. (6,7) Moreover, MP in the marine environment has been identified as a source of dissolved organic carbon (DOC), thereby intensifying ocean acidification. (8) Under increasingly acidified conditions, the degradation of plastic debris is enhanced, resulting in the generation of additional MP. (9) This self-reinforcing cycle underscores highlights the escalating severity of MP pollution and OA in future marine environments and underscores the imperative to evaluate their impacts on marine biogeochemical cycles and global climate.
Dimethylsulfide (DMS) is the most abundant volatile biogenic sulfur compound in the ocean, contributing a sea-to-air flux of 27.1 Tg S per year and accounting for 50% to 80% of marine sulfur emissions. (10) Atmospheric mixing ratios of DMS in marine environments typically range from tens to hundreds of parts per trillion (pptv), which is approximately 3 orders of magnitude lower than its concentration in seawater (nmol L–1). (11) Once emitted into the atmosphere, DMS undergoes rapid reactions with radicals, resulting in an atmospheric lifetime of approximately 1 to 2 days. The oxidation products of DMS play a crucial role in both the growth of existing aerosol particles and the formation of new particles, ultimately giving rise to nonsea-salt sulfate (nss-SO42–) aerosols. (12) These aerosols can directly scatter solar radiation or indirectly enhance the abundance of cloud condensation nuclei, thereby influencing cloud microphysical properties and modifying the global radiation budget. (13) The radiative forcing of DMS-derived aerosols is estimated to range from −1.7 to −2.3 W m–2, (14,15) a magnitude comparable to the positive radiative forcing from anthropogenic CO2 emissions (1.83 ± 0.2 W m–2). (16) Notably, short-lived polybrominated methane compounds, specifically bromoform (CHBr3) and dibromomethane (CH2Br2), serve as the main sources of bromine radicals in the atmosphere, which could contribute up to 30% of the DMS oxidation in the open sea. (17) Moreover, bromine radicals arising from CHBr3 and CH2Br2 could reduce the number of cloud condensation nuclei derived from DMS by decreasing the conversion efficiency of DMS to SO2 or shortening the lifetime of DMS, thereby weakening the climate-cooling effect of DMS. (18) The climate-active atmospheric DMS, CHBr3, and CH2Br2 are primarily emitted from marine sources, with production largely by phytoplankton and bacteria in the euphotic zone. (19) MP pollution and OA have been shown to impact the growth, community structure, and physiological behaviors of marine microbes, thereby altering the biogeochemical cycles of key elements in the ocean. (20,21) However, the influence of MP in future acidified marine environments on the production of climate-active gases like DMS, CHBr3, and CH2Br2 has not been explored, limiting our understanding of the potential climate impacts of MP pollution.
MP is recognized as a stressor for phytoplankton, with its physical collisions, shading effects, and leachates impacting the abundance and community structure of phytoplankton. (22) Additionally, OA exerts negative effects on physiological processes in phytoplankton, such as calcification and extracellular enzyme activity, particularly when combined with other environmental stressors. (23) Phytoplankton represent the major source of dimethylsulfoniopropionate (DMSP) and its cleavage product DMS in marine systems, (24) and thus the combined inhibitory impacts of MP and OA on phytoplankton physiology are expected to inhibit DMS production. The primary biogenic production pathway of polybrominated methane involves the reaction of bromoperoxidase (BrPO) with bromide ions in the presence of hydrogen peroxide (H2O2), yielding hypobromous acid (HOBr), which then reacts with dissolved organic matter (DOM) to form unstable intermediates that decompose into polybrominated methane. (25) Studies indicate that MP can leach DOM or increase the production and release of DOM by phytoplankton, (26,27) which serves as a substrate for the production of polybrominated methane. Since BrPO exhibits optimal activity at approximately pH 6.5, (28) the shift toward lower pH under OA could enhance BrPO-mediated bromination. Thus, both MP pollution and OA could potentially increase the production of polybrominated methane. Based on these insights, we hypothesize that MP pollution in future acidified marine environments could inhibit DMS production and enhance the production of CHBr3 and CH2Br2, thereby weakening the climatic cooling effect of DMS...
Dimethylsulfide (DMS) is the most abundant volatile biogenic sulfur compound in the ocean, contributing a sea-to-air flux of 27.1 Tg S per year and accounting for 50% to 80% of marine sulfur emissions. (10) Atmospheric mixing ratios of DMS in marine environments typically range from tens to hundreds of parts per trillion (pptv), which is approximately 3 orders of magnitude lower than its concentration in seawater (nmol L–1). (11) Once emitted into the atmosphere, DMS undergoes rapid reactions with radicals, resulting in an atmospheric lifetime of approximately 1 to 2 days. The oxidation products of DMS play a crucial role in both the growth of existing aerosol particles and the formation of new particles, ultimately giving rise to nonsea-salt sulfate (nss-SO42–) aerosols. (12) These aerosols can directly scatter solar radiation or indirectly enhance the abundance of cloud condensation nuclei, thereby influencing cloud microphysical properties and modifying the global radiation budget. (13) The radiative forcing of DMS-derived aerosols is estimated to range from −1.7 to −2.3 W m–2, (14,15) a magnitude comparable to the positive radiative forcing from anthropogenic CO2 emissions (1.83 ± 0.2 W m–2). (16) Notably, short-lived polybrominated methane compounds, specifically bromoform (CHBr3) and dibromomethane (CH2Br2), serve as the main sources of bromine radicals in the atmosphere, which could contribute up to 30% of the DMS oxidation in the open sea. (17) Moreover, bromine radicals arising from CHBr3 and CH2Br2 could reduce the number of cloud condensation nuclei derived from DMS by decreasing the conversion efficiency of DMS to SO2 or shortening the lifetime of DMS, thereby weakening the climate-cooling effect of DMS. (18) The climate-active atmospheric DMS, CHBr3, and CH2Br2 are primarily emitted from marine sources, with production largely by phytoplankton and bacteria in the euphotic zone. (19) MP pollution and OA have been shown to impact the growth, community structure, and physiological behaviors of marine microbes, thereby altering the biogeochemical cycles of key elements in the ocean. (20,21) However, the influence of MP in future acidified marine environments on the production of climate-active gases like DMS, CHBr3, and CH2Br2 has not been explored, limiting our understanding of the potential climate impacts of MP pollution.
MP is recognized as a stressor for phytoplankton, with its physical collisions, shading effects, and leachates impacting the abundance and community structure of phytoplankton. (22) Additionally, OA exerts negative effects on physiological processes in phytoplankton, such as calcification and extracellular enzyme activity, particularly when combined with other environmental stressors. (23) Phytoplankton represent the major source of dimethylsulfoniopropionate (DMSP) and its cleavage product DMS in marine systems, (24) and thus the combined inhibitory impacts of MP and OA on phytoplankton physiology are expected to inhibit DMS production. The primary biogenic production pathway of polybrominated methane involves the reaction of bromoperoxidase (BrPO) with bromide ions in the presence of hydrogen peroxide (H2O2), yielding hypobromous acid (HOBr), which then reacts with dissolved organic matter (DOM) to form unstable intermediates that decompose into polybrominated methane. (25) Studies indicate that MP can leach DOM or increase the production and release of DOM by phytoplankton, (26,27) which serves as a substrate for the production of polybrominated methane. Since BrPO exhibits optimal activity at approximately pH 6.5, (28) the shift toward lower pH under OA could enhance BrPO-mediated bromination. Thus, both MP pollution and OA could potentially increase the production of polybrominated methane. Based on these insights, we hypothesize that MP pollution in future acidified marine environments could inhibit DMS production and enhance the production of CHBr3 and CH2Br2, thereby weakening the climatic cooling effect of DMS...
A cartoon about the overall effects:
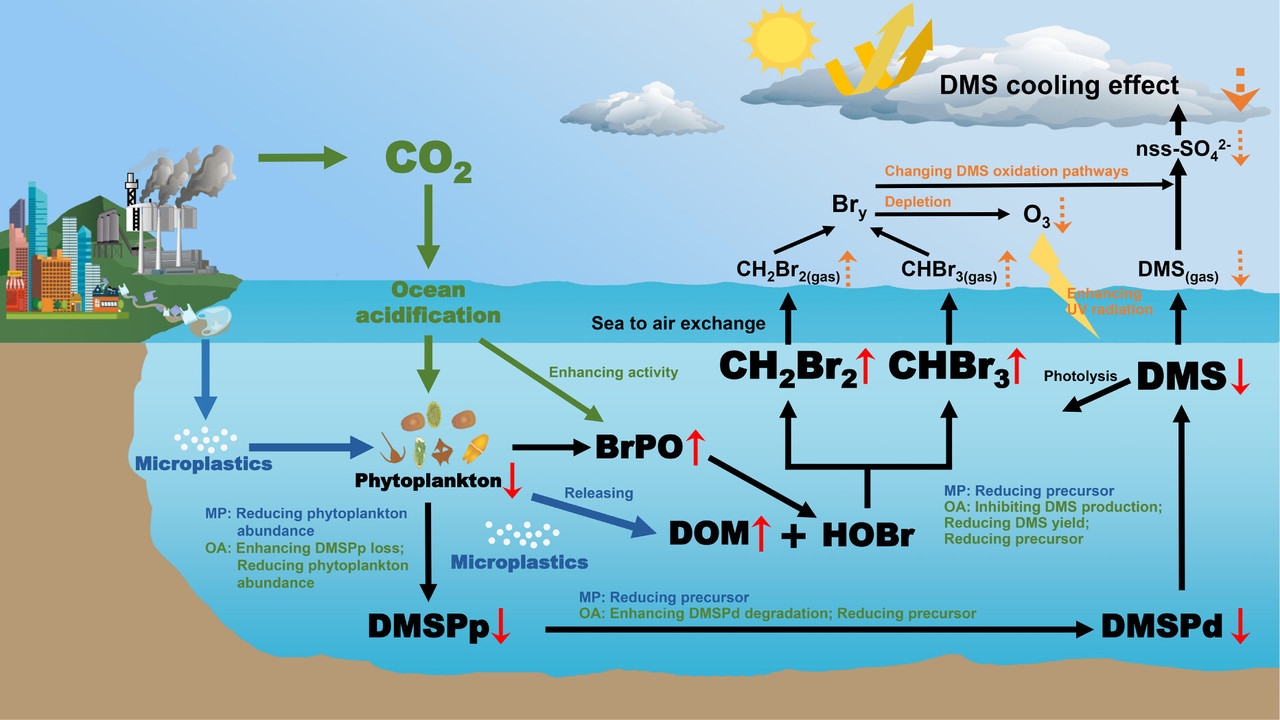
The caption:
Figure 4. Schematic overview illustrating the impacts of MP addition and OA on the production processes of DMS, CHBr3, and CH2Br2, and their potential climate feedbacks. The blue and green text indicate the observed effects of MP addition and OA on relevant pathways, respectively. Red arrows represent observed results from this experiment, while orange arrows indicate extrapolated processes based on the results of this study and previous literature.
Some additional fun text:
...Since the signing of the Montreal Protocol, short-lived halocarbons, particularly CHBr3 and CH2Br2, have played a pivotal role in stratospheric ozone depletion, with their impact on ozone radiative effects 3.6 times greater than that of long-lived halocarbons. (82,83) The simulations for the end of the 21st century under scenarios of OA and MP pollution showed significant increases in the concentrations of CHBr3 and CH2Br2 by 133% and 45%, respectively (Figure 3b, c), enhancing their atmospheric concentrations and promoting ozone depletion. A critical function of atmospheric ozone is to reflect UV radiation that reaches the Earth’s surface, which is the primary wavelength driving the photolysis of DMS. Seawater collected at station S10-1 for the ship-based microcosm experiment was also used in in situ incubation experiments to quantify the initial DMS removal pathways. The results indicated that photolysis, microbial consumption, and sea-air diffusion contributed 48%, 43%, and 9%, respectively, to the removal of DMS in surface seawater at this location (Figure 3e). The photolysis rate of DMS in surface seawater was at 1.62 nmol L–1 d–1, with UVA, UVB, and visible light contributing 39%, 46%, and 15% to the photolysis process, respectively (Figure 3f). This demonstrates that photolysis was a crucial factor in the removal of DMS from surface layers, with UV radiation predominantly driving the photodegradation process at this site. Therefore, based on the results of this experiment, the increased levels of CHBr3 and CH2Br2 in seawater due to the addition of MP and OA could enhance UV radiation by reducing atmospheric ozone levels. This enhancement in turn could strengthen the photolysis process of DMS in surface seawater, leading to further reductions in DMS concentrations...
While we are distracted by the accelerating fall of the United States in an orgy of anti-intellectualism, the planet as a whole is undergoing rapid decay.
5 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies