More Lipstick on the Gas Pig: Dry Reforming Methane with "Solar" Thermal Energy. [View all]
The paper I'll discuss in this post is this one: Photothermocatalytic Dry Reforming of Methane for Efficient CO2 Reduction and Solar Energy Storage Shaowen Wu, Yuanzhi Li, Qianqian Hu, Jichun Wu, and Qian Zhang ACS Sustainable Chemistry & Engineering 2021 9 (35), 11635-11651.
You here from time to time from people with rather glib wishful thinking that hydrogen is so called "green" energy because its combustion product is "only" water.
Despite oodles and oodles and oodles and oodles of papers in the literature, and hundreds of thousands of internet posts about "green" hydrogen made with so called "renewable energy," where so called "renewable energy" is defined largely by solar and wind energy, the overwhelming amount of hydrogen produced on this planet is produced from the reformation of dangerous natural gas at high temperatures, the temperatures being provided by burning dangerous natural gas and dumping the dangerous natural gas waste carbon dioxide into the atmosphere without restriction.
That's a fact.
Facts matter.
So called "renewable energy cannot survive without access to dangerous fossil fuels and this paper, which bills itself as an energy storage paper - energy storage is by definition an exercise in wasting energy (unless it recovers energy that would have been wasted anyway) - is no exception.
"DRM" in the context of this paper, is referred to as "dry reforming of methane." Dry reforming is a well known technology in which carbon dioxide is utilized as an oxidant, and in turn is converted to carbon monoxide. Dry reforming is possible with many carbon compounds; the dangerous fossil fuel methane, the chief component of dangerous natural gas just happens to seem cheap, because the real costs will be paid by future generations, about whom the current generation couldn't give a rat's ass.
This paper is, about solar reforming. In these times any appalling and unsustainable practice can be greenwashed simply by sticking the word "solar" on it and assuming most people are too lazy to see through it, generally a good bet for advertisers.
From the text:
Light-driven thermochemical CO2 splitting involves two steps such as the decomposition of appropriate metal oxides at very high temperature (usually above 1600 °C) and the oxidation of the reduced metal oxides by CO2 to produce CO by using very high concentrated solar illumination (e.g., 1500 suns).(13,14) The major challenge is the difficulty of realizing the decomposition of metal oxides with moderate concentrated solar illumination due to the thermodynamic limit of metal oxide decomposition. Photocatalytic CO2 reduction and DRM involves CO2 reduction by electrons and the oxidation of H2O or organic compounds (e.g., CH4 for DRM) by holes on semiconductor photocatalysts. Extensive works have been devoted to improve the fuel production rate (rfuel) and light-to-fuel efficiency (η
I've been hearing all kinds of wonderful stuff about solar thermal technologies my whole adult life by the way, and I'm not by any stretch young. Nevertheless, despite all this cheering, we saw concentrations of 420 ppm of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere this spring, less than ten years after we first saw 400 ppm.
Facts matter.
The authors continue:
Although most of what is written in this paper is disturbing, to me at least, there is some useful science in it, a nice mini-review of sorts of dry reforming.
To wit:
With these ideas in mind, a gas-phase reactor for photothermocatalytic DRM (Figure 1A) was designed.(87) Focused illumination from a 500 W Xe lamp was used to drive the reaction. No additional heater is used besides the Xe lamp. A nanocomposite of Pt nanocrystals (with average particle size of 2.1 nm) partially confined in mesoporous CeO2 nanorods (Pt/CeO2-MNR) was prepared. A known amount of the catalyst was put in a thermal insulation sample holder to reduce heat loss. A feed stream of CH4 and CO2 was continuously fed into the reactor.
Note the Xe lamp. One would think that more than half a century into wishful thinking about how solar energy would save us - many of us having bet the future of humanity on this unproved supposition - that there would be lots and lots and lots of handy solar thermal reactors lying around with which to do experiments. This however, would involve scientists working on grants, some working for Ph.Ds. to be able to work only when the sun is shining brightly and hotly. This would limit the lab time, so xenon lamps are used as a surrogate.
This should tell you something.
Don't worry. Be happy. Let's just look at the pictures from the text and feel all fuzzy inside.
The introductory cartoon:
A picture of the apparatus with the xenon lamp:
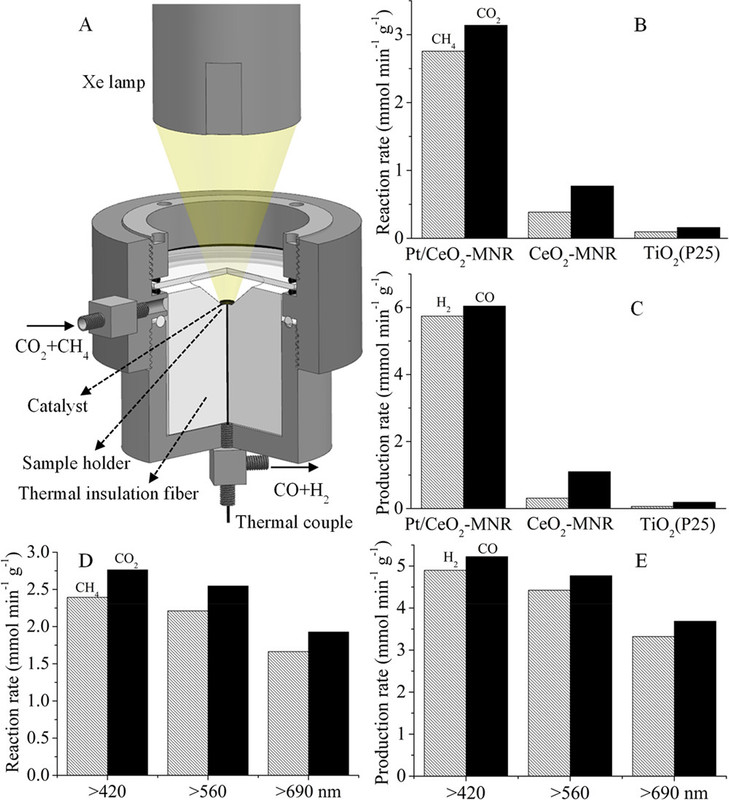
The caption:
Reference 87 is this one: Solar-light-driven CO2 reduction by methane on Pt nanocrystals partially embedded in mesoporous CeO2 nanorods with high light-to-fuel efficiency† (Li et al, : Green Chem., 2018, 20, 2857)
The work was performed in China, and the odds are overwhelming that the Xenon lamp was produced using electricity generated in a coal fired plant.
Nevertheless if you want to do so, and many people do like to read this kind of garbage, you can head over to read rhetoric by a blatantly dishonest bunch of disingenuous horseshit by an anti-nuke telling us how, in China, so called "renewable energy" had defeated the nuclear industry, using all kinds of misleading charts and graphs designed around the assumption you're stupid:
An Exercise in Bad Thinking: Nuclear In China Shows Clear Scalability Winners The barely literate dweeb who wrote this piece and published it in the year we first saw 420 ppm of the dangerous fossil fuel waste carbon dioxide in the atmosphere, less than 10 years after we first saw 400 ppm of the same waste now setting the planet afire, like most anti-nukes, is interested in so called "renewable energy" as an attack on the only climate change tool that actually works, nuclear energy, and couldn't give a rat's ass about dangerous fossil fuels, the use of which is growing, not falling. His name is Michael Bernard, and he wants you to know that he knows Leonardo Di Caprio and that he's willing to consult for you, presumably for a fee.
If you don't know what you're talking about, make stuff up.
His triumphal account includes a picture which I personally find disgusting, of a huge stretch of land covered by soon to be electronic waste, which may be, for a short period of a day, be able to produce as much power as a nuclear plant on less than 15 acres can produce reliably, night and day, 24/7, 365.25 days per year.
Land use changes are second only to the indiscriminate dumping of dangerous fossil fuel waste in driving carbon dioxide concentrations ad climate change.
Feel Free to call on him if you think the laws of thermodynamics should be repealed.

The caption:
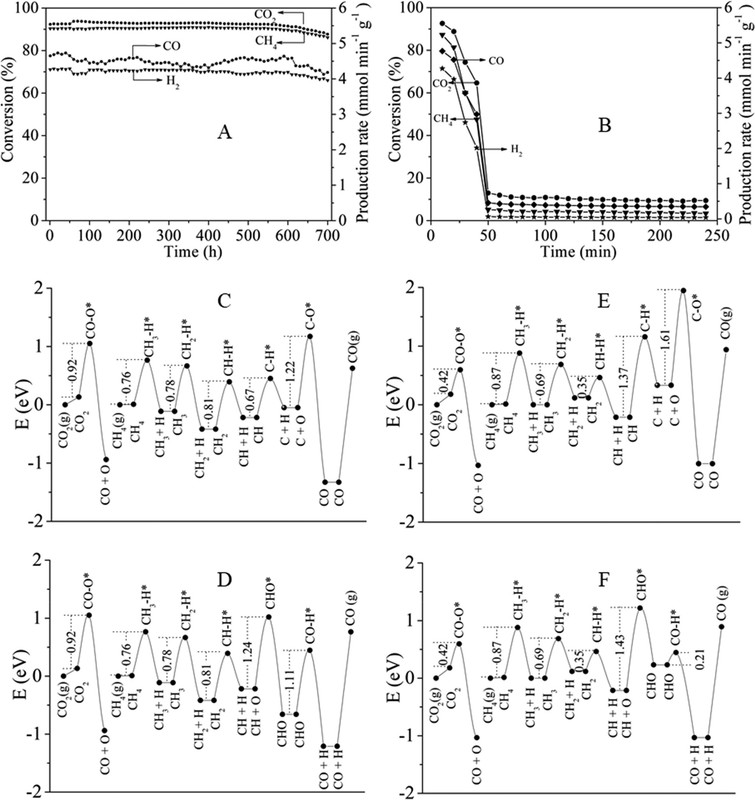
Figure 2. Optical absorption spectra of Pt/CeO2-MNR and CeO2-MNR (A). Time course of production rates for photocatalytic DRM on Pt/CeO2-MNR under UV–vis–IR illumination at near room temperature (B). Schematically illustrated light-driven thermocatalytic DRM on Pt/CeO2-MNR (C). The Teq values of the samples and the sample holder under focused UV–vis–IR illumination (D). Thermocatalytic activity of Pt/CeO2-MNR for DRM in the dark at different temperatures (E and F).(87)
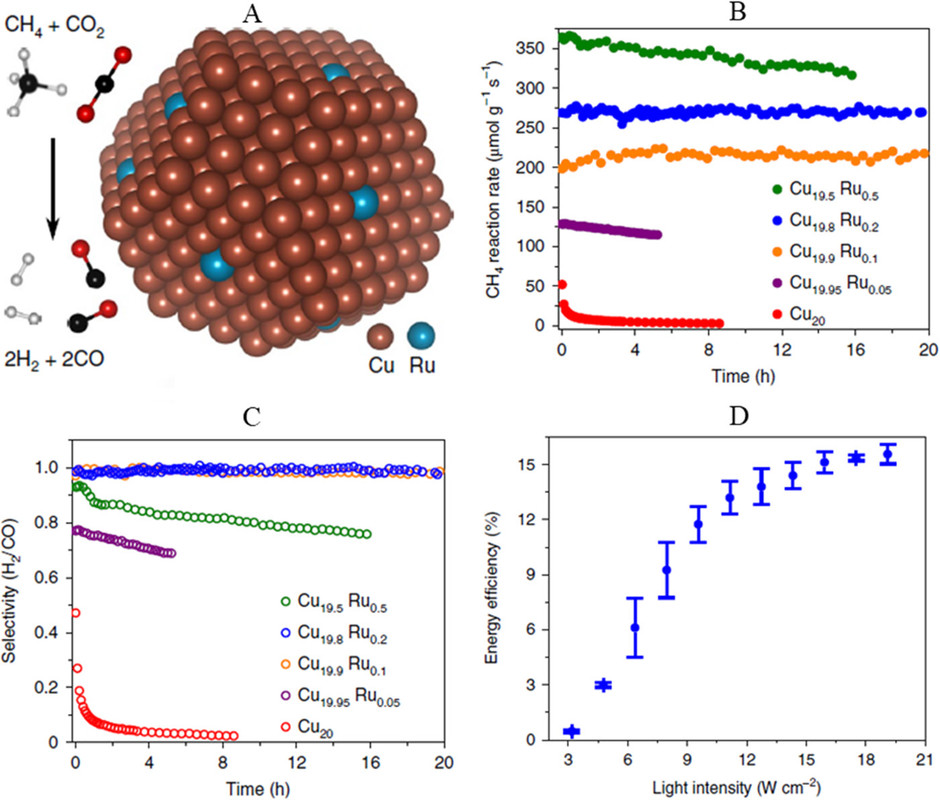
The caption:
Ruthenium is a relatively rare element, but it can be obtained from used nuclear fuels as it is a fission product.

The caption:
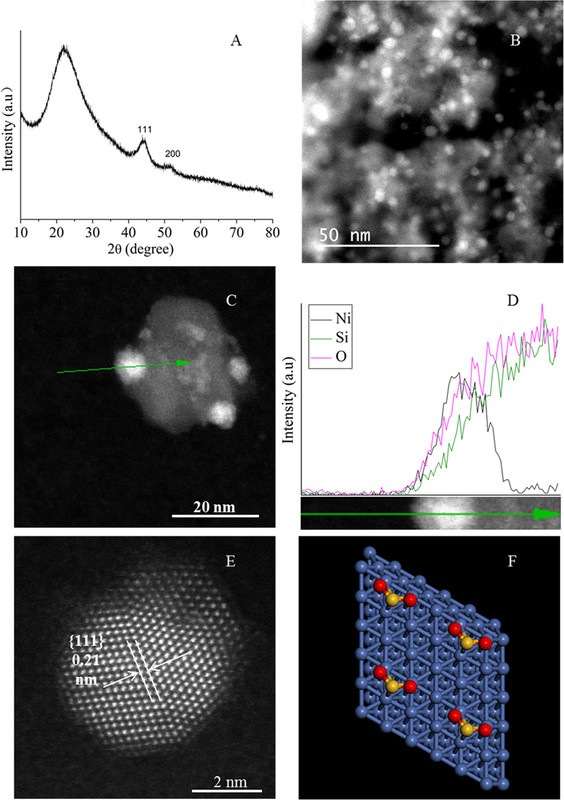
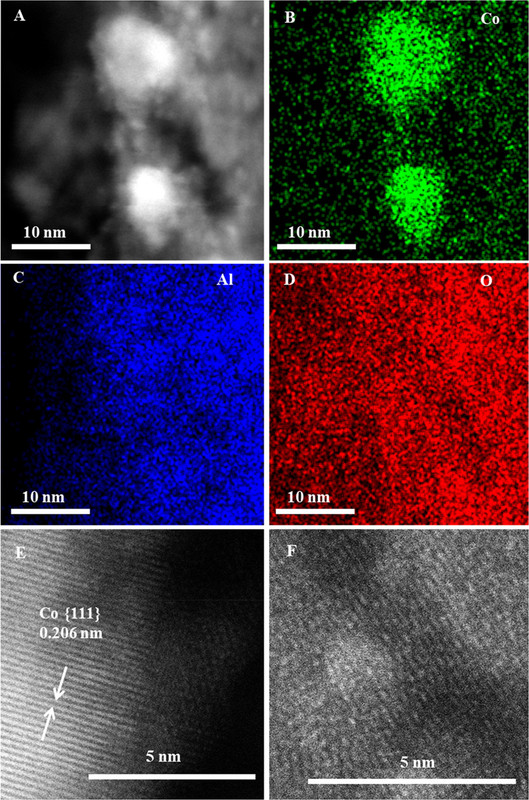
The caption:
The caption:
The caption:
The caption:
The caption:
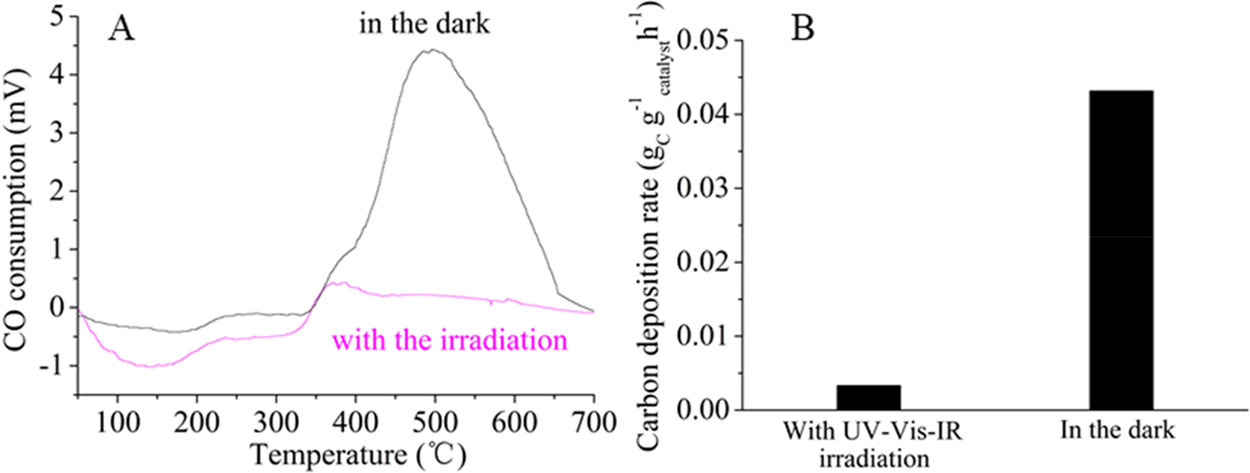
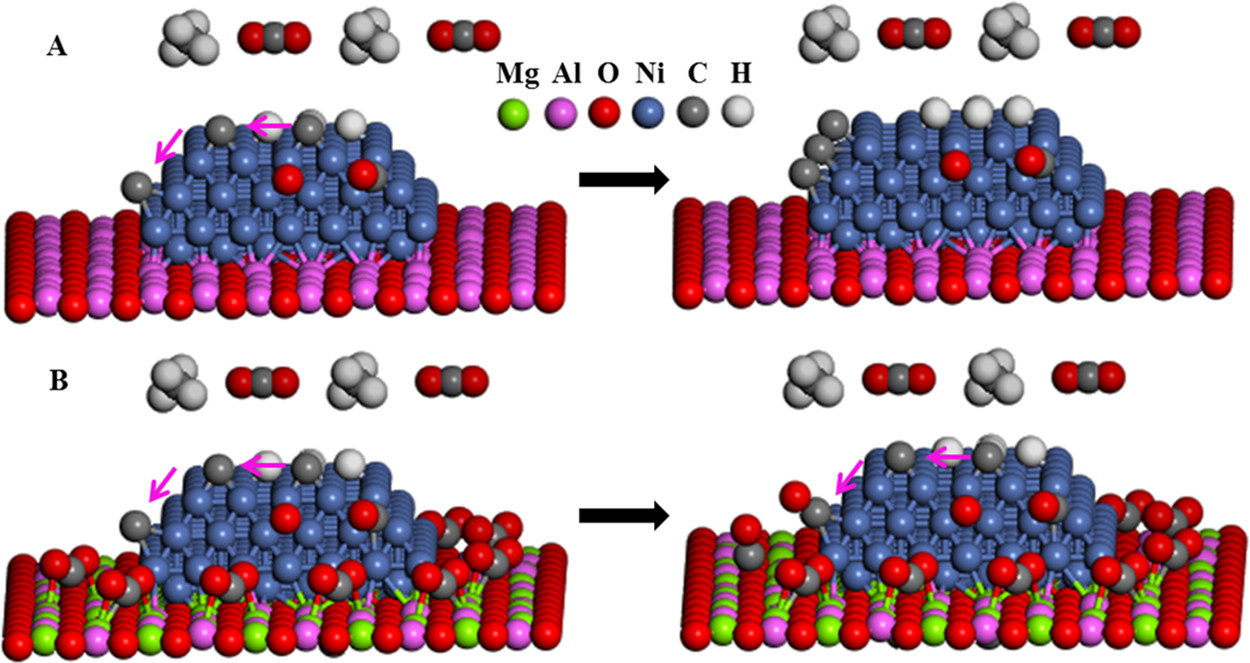
The caption:
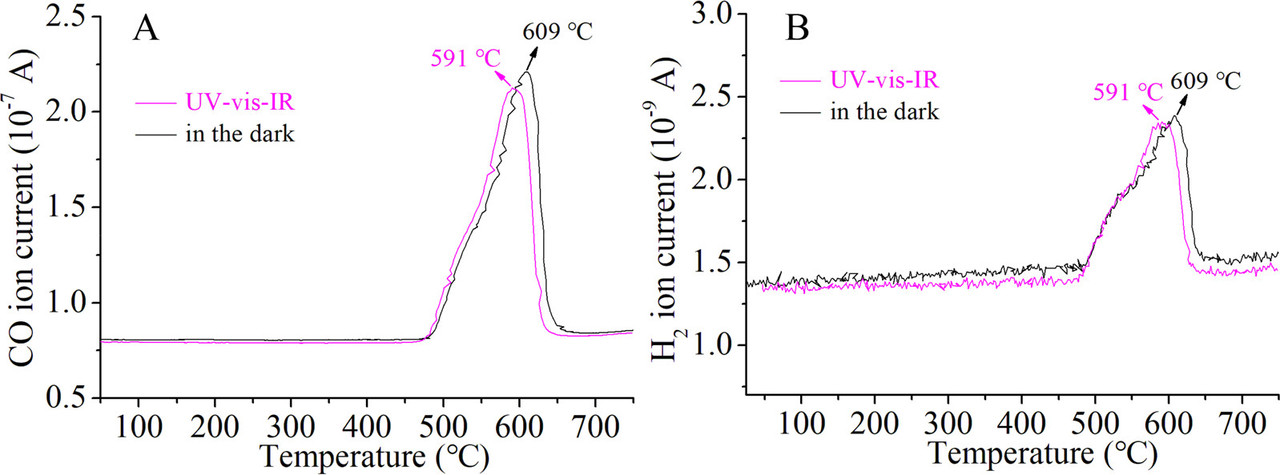
The caption:
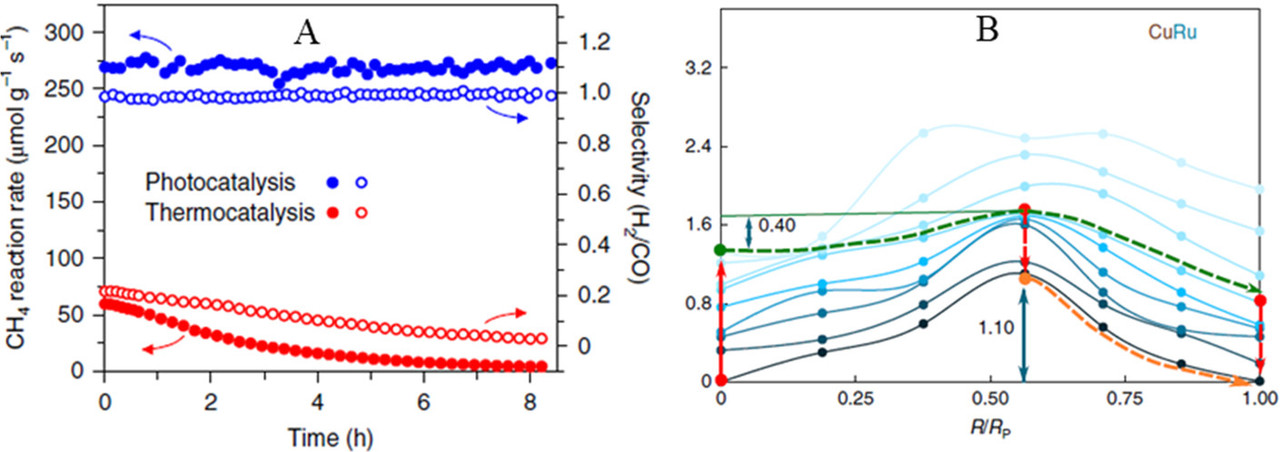
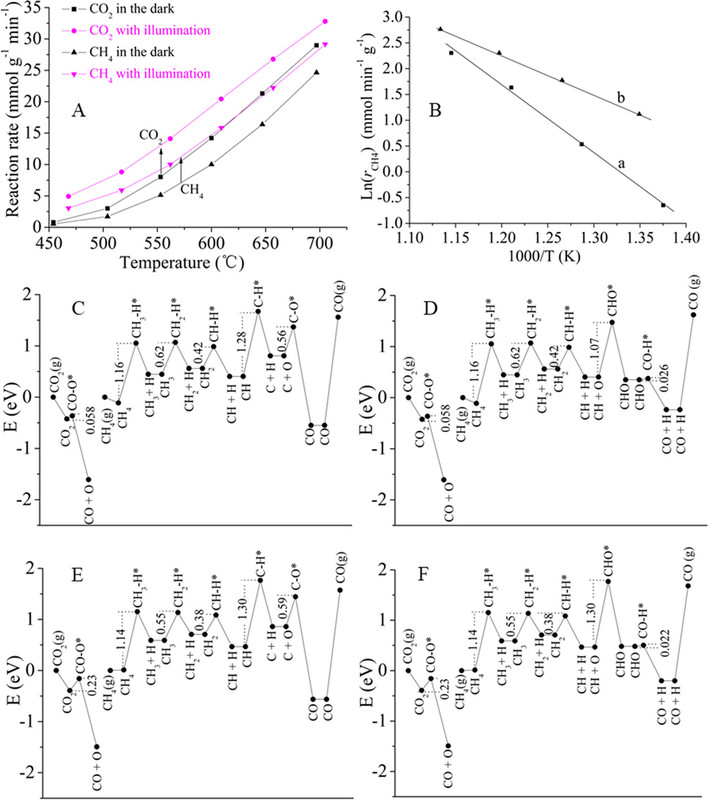
The caption:
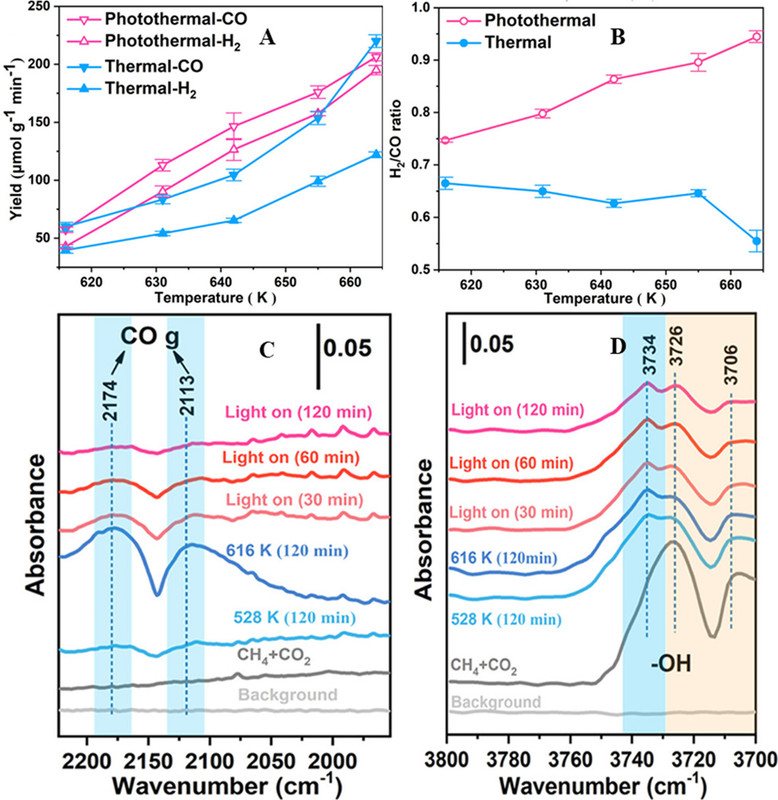
The caption:
Gallium is a critical element. The world will run out of methane, the sooner the better from my perspective, but the costs of using this methane in our generation will remain with humanity as long as humanity exists.
Have a nice evening.